Increasing demands for natural gas, coupled with the urgent need to reduce greenhouse gases, have resulted in significant research into the production of synthetic natural gas (SNG) – a potentially vital energy carrier in the future.
Hydrogenation of CO2 – commonly known as the ‘methanation reaction’ - represents a viable technique for the fixation of CO2.
To explore this further, nickel supported on yttrium oxide and promoted with cobalt was prepared by using the wet-impregnation method. This was then characterized using XRD, FTIR, SBET, XPS, TPR, and HRTEM/EDX.
An examination was undertaken into CO2 hydrogenation over the Ni/Y2O3 catalyst, aiming to assess how this compared against Co-Ni/Y2O3 catalysts: Co% = 10 and 15 wt/wt.
Carbon dioxide hydrogenation was performed using a homemade fixed bed tubular reactor working at atmospheric pressures.
An electric furnace equipped with a programmable temperature controller was used to heat the quartz silica reactor, and a total of 2 gm of the fresh catalyst was diluted with silicon carbide (SiC). This resulted in a 5 cm bed height packed in the middle of the reactor.
A K-type thermocouple placed in the center of the catalyst bed facilitated temperature monitoring.
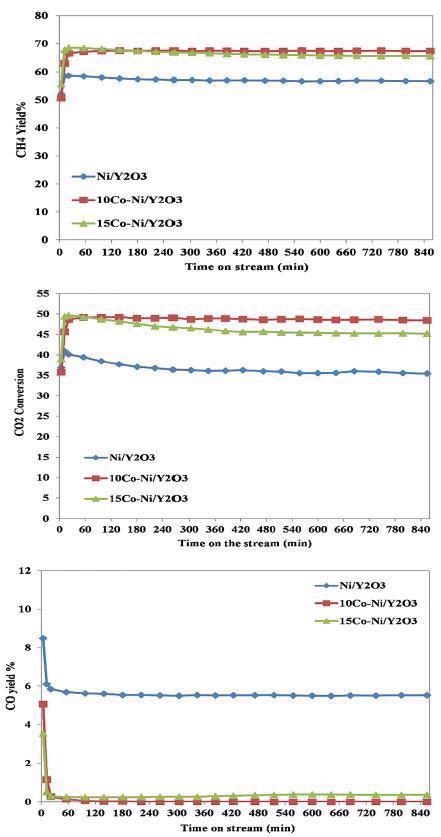
CO2 conversion, CH4 yield%, and CO yield% during the methanation reaction over yttrium-based catalysts. Image Credit: Hiden Analytical
Prior to the catalytic test, samples were all activated in situ using a 30 mL per minute flow of pure hydrogen at atmospheric pressure. This was done for 2 hours at 600 °C.
Following the reduction, catalysts were cooled, and a flow of premixed gas at a molar ratio CO2/H2/Ar = 1/4/5 with GHSV of 6000 ccg-1h-1 was introduced through the catalysts gradually.
The temperature was then increased to 350 °C at a 3-hour reaction time. The Hiden Analytical QGA was used to analyze gaseous reaction products on-line.
The selectivity of CO2, H2, CH4 and CO was detected via the gas analyzer utilizing a matrix equation to correct the overlapped detection values from the m/z of 28 (CO), 44 (CO2), 2 (H2), and 16 (CH4).
The CH4 yield was found to reach 67%, while CO2 conversion was determined to extend to 48.5% with CO traces over the 10Co-Ni/Y2O3 catalyst. This process promotes the direct methanation reaction mechanism.
However, the reaction mechanism over Ni/Y2O3 catalyst displayed different behaviors, as opposed to the behavior over bi-metal catalysts. The rise in steam reforming of the methane reaction was linked to the consumption of methane and an increase in H2 and CO formation; while occurring at an identical reaction temperature.
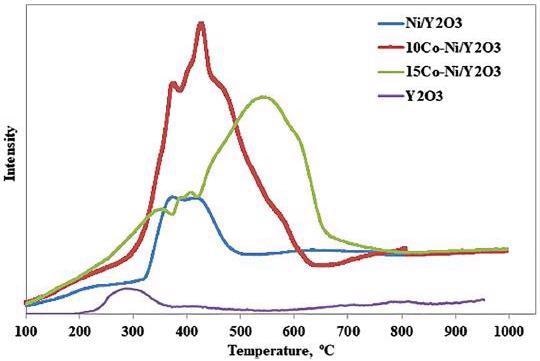
H2-TPR of the prepared catalysts. Image Credit: Hiden Analytical
Reference
“CO2 Valorization into Synthetic Natural Gas (SNG) using a Co-Ni bimetallic Y2O3 Based Catalysts”, Int. J. Chem. React. Eng (2021) 19, 6, 571-583 doi.org/10.1515/ijcre-2020-0163
Acknowledgments
Produced from materials originally authored by Radwa A. El-Salamony from the Egyptian Petroleum Research Institute (EPRI).

This information has been sourced, reviewed and adapted from materials provided by Hiden Analytical.
For more information on this source, please visit Hiden Analytical.