Edible oils are staple foods and irreplaceable raw materials for many industries. Apart from their role in food preparation and processed food manufacturing, they find applications in cosmetics, biofuels, oleochemicals, and pharmaceuticals.
To ensure consumer safety and environmental protection, standards and guidelines oversee the continual assessment of their quality and safety parameters throughout the supply chain.
Given the sheer volume of edible oil to be analyzed and the complexity of production, industrial laboratories of food and drug manufacturers, as well as laboratories of government agencies, face the challenge of obtaining reliable analytical data for high sample quantities in the shortest possible time.
In addition to metal and heavy metal traces, sulfur, nitrogen, and chlorine often need to be determined. These elements typically do not naturally occur in edible oils but enter as impurities, influencing the production process, quality, and characteristics of the final product and impacting odor, color, taste, and shelf life.
Certain elements even contribute to the creation of harmful substances. Examining these factors is crucial not just for quality but also for safety purposes. Their concentration often falls within the ppb or low ppm range.
Sulfur holds a unique position; while considered undesirable as an impurity in many oils, it can serve as a quality indicator in others.
Such oils are often obtained from the seeds of cruciferous plants (brassicaceae). They naturally contain organically bound sulfur in the form of glucosinolates, which form allyl thiocyanates during their enzymatic degradation process.
These substances not only impart an intriguing flavor to the oil but also boast antiseptic, disease-preventive, and other health-related effects. Mustard seeds, cabbage, radish, cress, or capers are well-known examples, with their oils employed in natural medicine and dietary supplements.
Determination of total sulfur as a sum parameter (TS) has proven useful in both tasks when rapid control of sulfur content is required. Elemental analysis coupled with UV-fluorescence detection is a suitable technique for this purpose and requires only a single, non-matrix-specific calibration for the quantification of sulfur.
This technique offers a broad measurement range across matrices of different viscosities. It has seen longstanding use in chemical and petrochemical industries and is extensively outlined in local and global standards, including ASTM D5453, which is particularly beneficial for challenging matrices like mineral oil.
Edible oils exhibit similar traits to mineral oil and related samples, such as high viscosity, elevated boiling points, and long-chain or saturated hydrocarbons. Thus, adapting the ASTM standard for mineral oil analysis can be easily applied to assess edible oils.
Chromatographic methods are recommended for more precise identification of the sulfur compound.
Here, a particular compound can be accurately quantified in the presence of all other sulfur-containing substances.
The following analysis of a representative spectrum of various vegetable oils shows how easily combustion elemental analysis can be adapted for the pretreatment-free, sensitive and precise determination of sulfur over the entire concentration range.
Materials and Methods
Samples and Reagents
- 14 edible oil samples from different processing stages (starting materials, intermediates, end products) and different production processes (cold pressed, solvent extracted, refined, hydrogenated) as well as a high purity reference oil (paraffine oil)
- Kit calibration solutions, c: 0.1 – 10 mg/L S, Analytik Jena GmbH+Co. KG, 402-889.070
- Kit calibration solutions, c: 10 – 100 mg/L S, Analytik Jena GmbH+Co. KG, 402-889.167
- Kit calibration solutions, c: 100 – 500 mg/L S, Analytik Jena GmbH+Co. KG, 402-889.164
Sample Preparation
Both the samples and standards underwent direct analysis without any prior sample treatment. Utilizing a heated supply system, such as a temperature-controlled liquid sampler, enabled the direct injection of samples using a syringe. Heating the samples to 75 °C notably decreased their viscosity.
This method is applicable for dealing with uniform liquids that have heightened viscosity. Another approach involves diluting the samples with an appropriate solvent to lower viscosity, enabling automatic sample delivery without requiring a specialized sampler. However, this specific strategy was not employed in the experiments detailed here.
Calibration
Before the measurements, the multi EA 5100 underwent calibration specifically for sulfur determination. This involved utilizing liquid standards composed of dibenzothiophene (S) in isooctane, spanning concentrations from 0.1 to 500 mg/L S, followed by conducting a blank correction.
The calibration data obtained was then cross-checked using certified reference standards.
An injection volume of 40 µL was utilized for both calibration and verification measurements. Figure 1 displays the calibration curve for the ultra-trace range, while its performance metrics are outlined in Table 1.
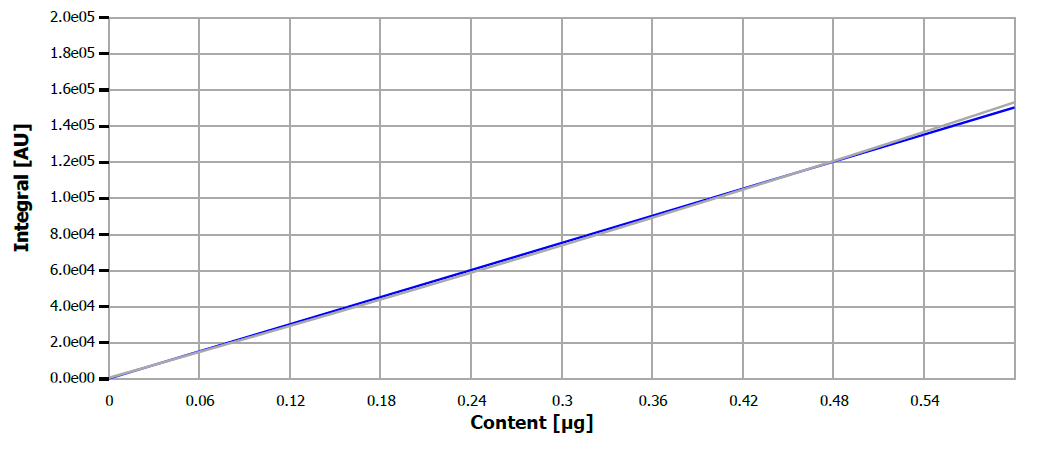
Figure 1. Calibration curve for sulfur. Image Credit: Analytik Jena US
Table 1. Calibration performance parameters. Source: Analytik Jena US
| Parameter |
Value |
| Injection volume |
40 μL |
| Concentration range |
0-0.4 μg S abs. |
| Detection limit |
5.99 μg/L |
| Linearity |
linear |
| Correlation coefficient |
0.9996 |
Instrumentation
A multi-EA 5100 analyzer equipped with a HiPerSens sulfur detector was used in horizontal operation mode. For automated sample introduction and transfer into the analyzer, the system was equipped with an automatic boat drive with flame sensor technology and with the temperature-controlled multi-matrix sampler MMS-T.
The MMS-T system is designed for versatility, offering cooling capabilities for the automated dosing of highly volatile, lightweight liquids, as well as heating features for the direct dosing of viscous, homogeneous liquids without the need for prior dilution.
The flame sensor guarantees seamless and matrix-optimized transfer and combustion of various materials.
A volume of 40 µL of the sample or standard was utilized for sulfur determination. Sample digestion was executed through efficient catalyst-free high-temperature combustion within a quartz tube.
The measurement process is controlled and fully automatically adapted to the needs of every matrix component by the flame sensor technology. This ensures matrix-independent, optimal results in the shortest possible time.
The process consists of two phases. Initially, the volatile components of the sample are evaporated within an inert gas stream, followed by the combustion of the resulting gaseous products in an oxygen-rich atmosphere.
In the subsequent phase, the denser, nonvolatile sample components and resulting pyrolysis products are fully oxidized in pure oxygen. An integrated auto-protection system ensures the highest operational safety by trapping particles and aerosols. Achieving a detection limit of 5 µg/L is quite feasible.
Method Parameters
For the analysis of liquid standards and oil samples, a method from the method library was used. The method parameters are summarized in Table 2. The injection volume for TS determination was standardized at 40 µL. Utilizing a temperature-controlled syringe enabled direct injection of the pure samples.
Throughout the duration of the analysis, both the syringe and the sample (on the sample tray) were maintained at a consistent temperature of 75 °C. This ensured direct injection, irrespective of the original sample viscosity, thereby circumventing time-consuming and error-prone dilution processes.
Table 2. Process parameters multi EA 5100 – horizontal operation. Source: Analytik Jena US
| Parameter |
Setting |
| Furnace temperature |
1050 °C |
| 2nd combustion |
60 s |
| Ar flow (1st phase) |
200 mL/min |
| O2 main flow |
200 mL/min |
| O2 flow (2nd phase) |
200 mL/min |
| Draw up speed |
2.0 μL/s |
| Injection speed |
2.0 μL/s |
| ABD mode |
automatic* |
* flame sensor controlled automatically optimized combustion
Evaluation Parameters
The evaluation parameters for detecting sulfur contents (TS) through UV fluorescence are consolidated in Table 3.
Table 3. Detection parameters for total sulfur determination (UVFD). Source: Analytik Jena US
| Parameter |
Setting |
| Max. integration time |
720 s |
| Start |
0.1 ppb |
| Stop |
0.1 ppb |
| Block |
7 |
Results and Discussion
The results obtained for both samples and standard solutions are consolidated in Table 4, representing the average values derived from three replicates. No issues arose during the analysis of the edible oil samples, and there were no indications of memory effects, possibly induced by the higher viscosity of the samples.
The low RSD (Relative Standard Deviation) values in sample measurements were akin to those of the measured standard solutions, affirming the high reproducibility of the results. Even for samples like almond oil, in which sulfur impurities occur only in the ultra-trace range, the quality of the results was convincing.
This indirectly supports the repeatability of the injection process and the effectiveness of the digestion process. Furthermore, aside from analyzing edible oil, this measurement method could apply to other homogeneous oils and similar matrices (such as UCO, tall oil, cod liver oil, and glycerol).
Table 4. Results of Total Sulfur determination for the edible oil samples and standards. Source: Analytik Jena US
| Sample |
cS [mg/L] |
RSD [%] |
| Mustard oil, native |
234 |
1.60 |
| Linseed oil |
40.8 |
0.50 |
| Mustard oil 2 |
14.4 |
0.07 |
| Mustard oil* |
3.86 |
1.95 |
| Rice bran oil |
3.67 |
2.53 |
| Walnut oil |
2.88 |
0.76 |
| Palm oil raw |
2.75 |
0.40 |
| Rapeseed (canola) oil |
1.85 |
1.48 |
| Sesame oil |
0.81 |
0.75 |
| Peanut oil |
0.76 |
0.85 |
| Coconut oil |
0.47 |
3.01 |
| Olive oil |
0.38 |
4.83 |
| Almond oil |
0.19 |
1.32 |
| Paraffin oil SA |
1.43 |
0.44 |
| Standard 0.51 mg/L S |
0.51 |
0.91 |
| Standard 5.00 mg/L S |
5.00 |
2.31 |
* refined
The experiments notably highlighted the impact of production and processing methods on sulfur-containing compound levels. This is evident in the comparison of three distinct mustard oil samples below.
The naturally pressed mustard oil retains a considerable amount of sulfur compounds, whereas the other two variations exhibit notably reduced concentrations. In the "mustard oil 2" sample, sulfur content is 16 times lower, and in the refined "mustard oil" sample, only trace amounts are detected.
Below are typical measurement curves illustrating selected oil samples and standard solutions.
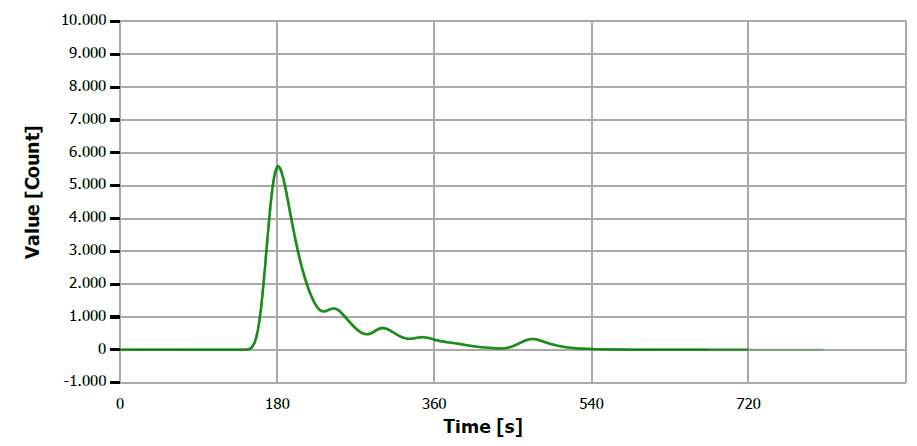
Figure 2. TS measuring curve for sample “lineseed oil”. Image Credit: Analytik Jena US
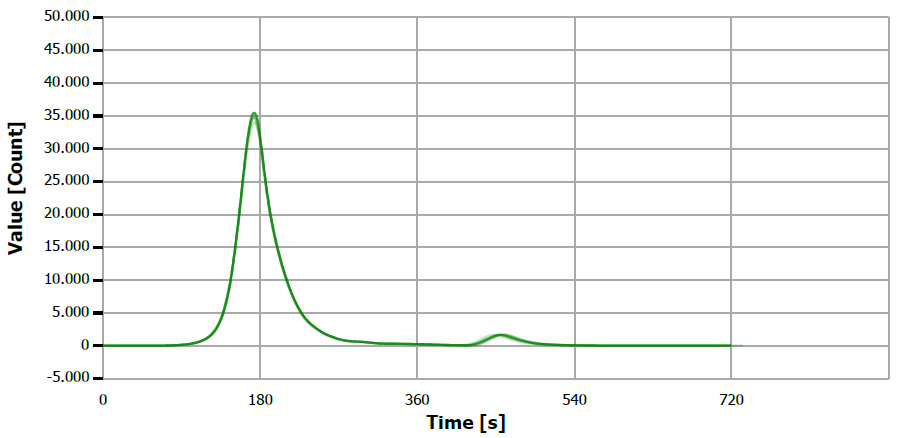
Figure 3. TS measuring curve for sample “mustard oil, native”. Image Credit: Analytik Jena US
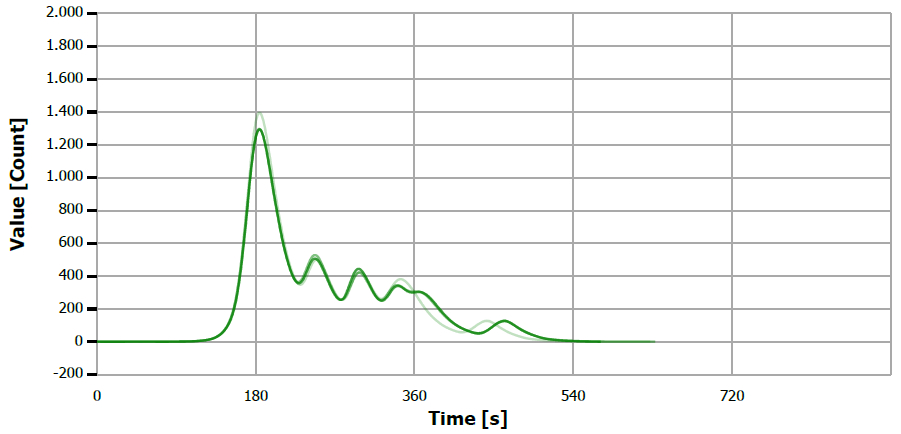
Figure 4. TS measuring curve for sample “mustard oil 2”. Image Credit: Analytik Jena US
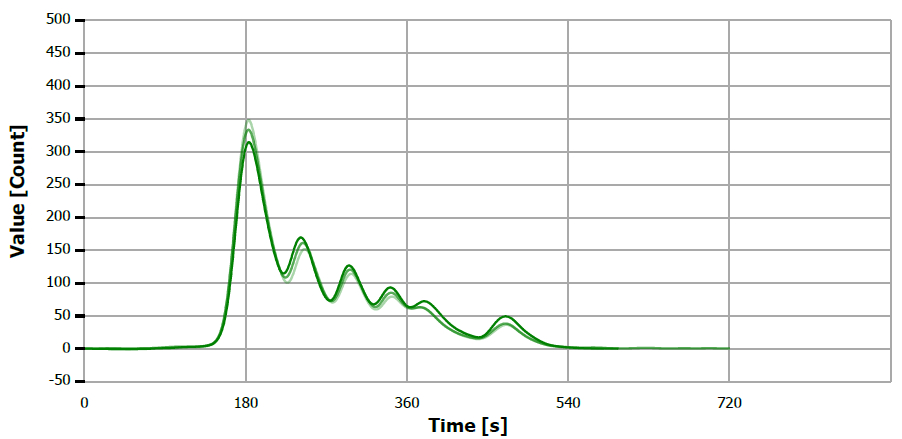
Figure 5. TS measuring curve for sample “mustard oil”. Image Credit: Analytik Jena US
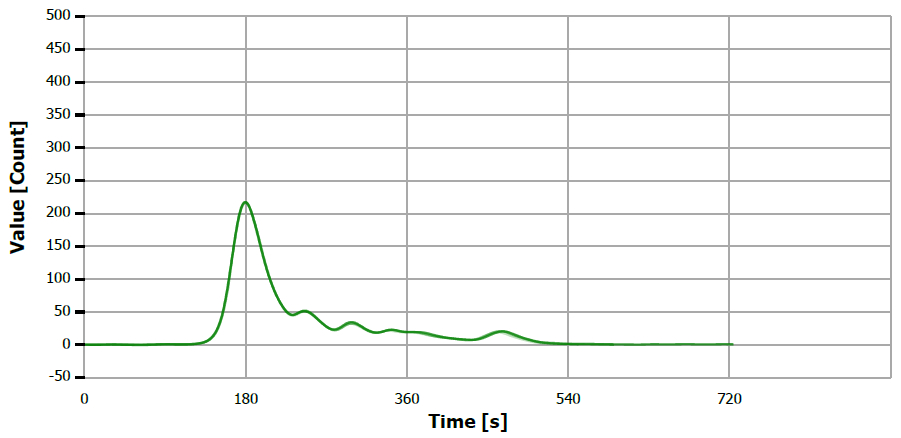
Figure 6. TS measuring curve for sample “rapeseed oil”. Image Credit: Analytik Jena US
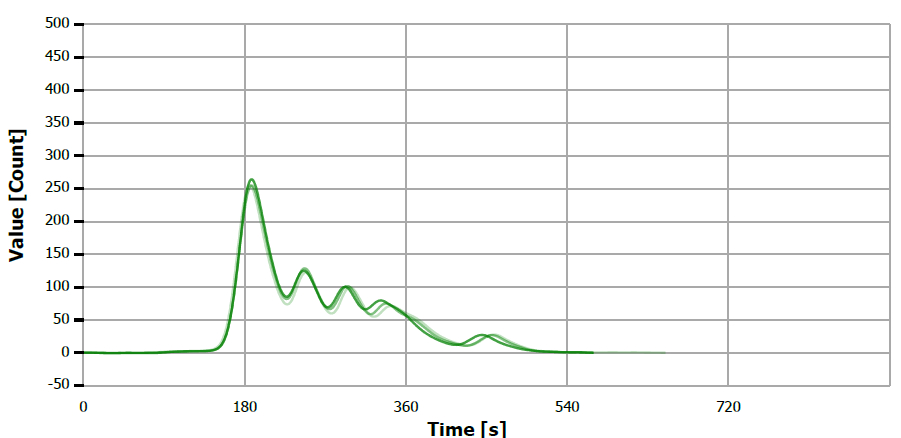
Figure 7. TS measuring curve for sample “walnut oil”. Image Credit: Analytik Jena US
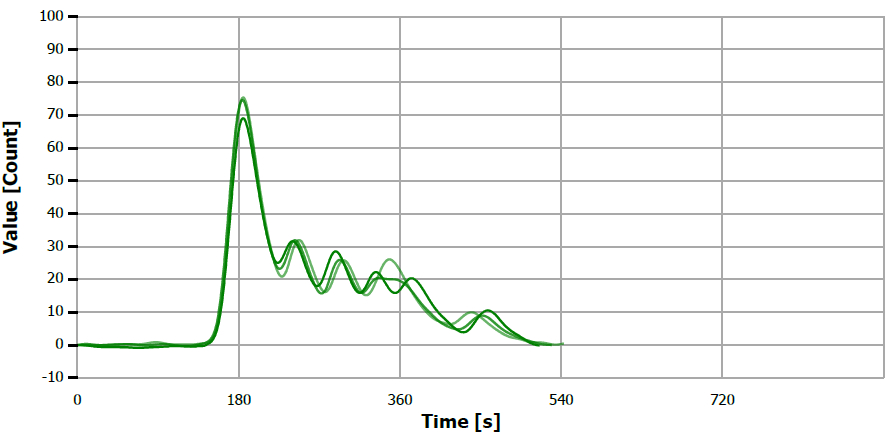
Figure 8. TS measuring curve for sample “sesame oil”. Image Credit: Analytik Jena US
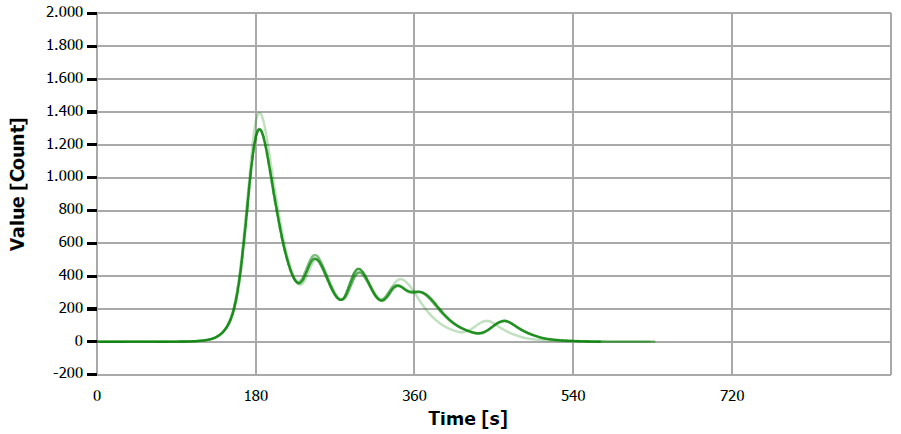
Figure 9. TS measuring curve for sample “palm oil”. Image Credit: Analytik Jena US
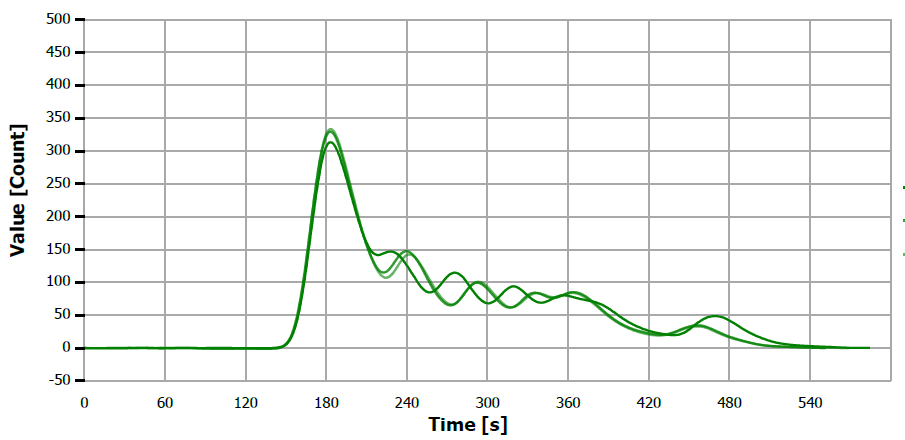
Figure 10. TS measuring curve for sample “rice bran oil”. Image Credit: Analytik Jena US
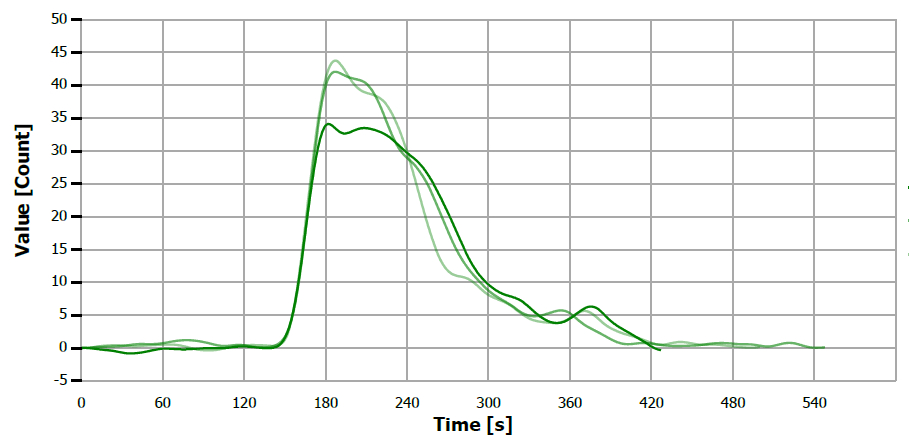
Figure 11. TS measuring curve for sample “coconut oil”. Image Credit: Analytik Jena US
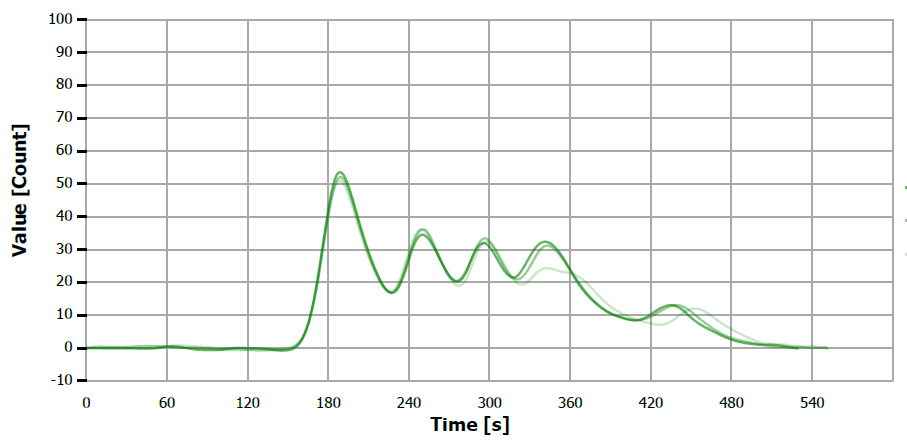
Figure 12. TS measuring curve for sample “peanut oil. Image Credit: Analytik Jena US
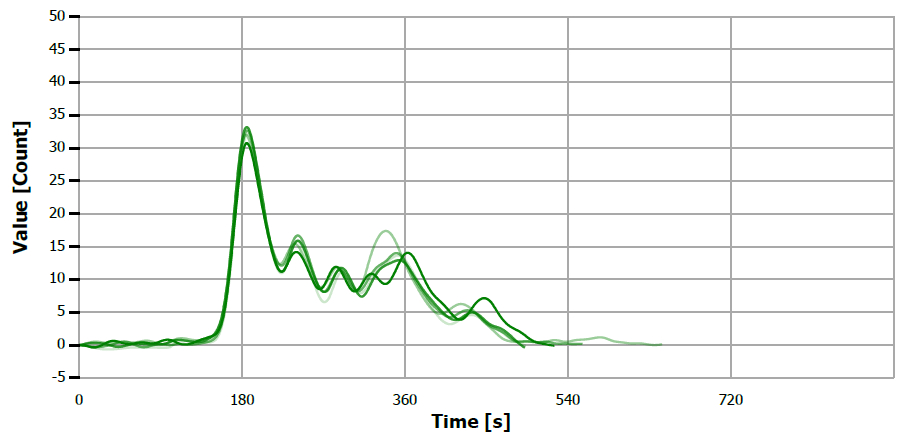
Figure 13. TS measuring curve for sample “olive oil”. Image Credit: Analytik Jena US
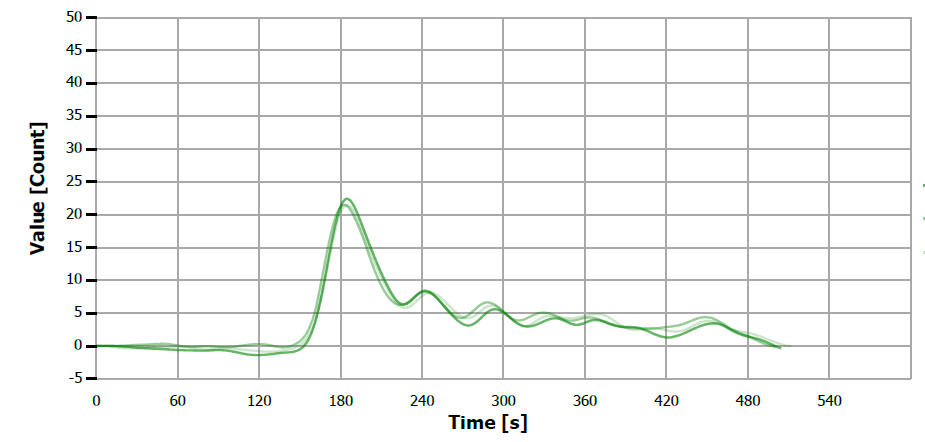
Figure 14. TS measuring curve for sample “almond oil”. Image Credit: Analytik Jena US
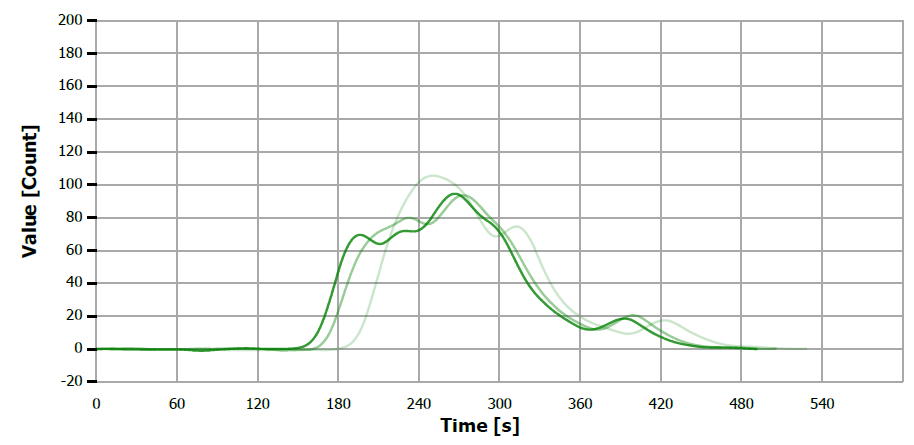
Figure 15. TS measuring curve for sample “paraffine oil”. Image Credit: Analytik Jena US
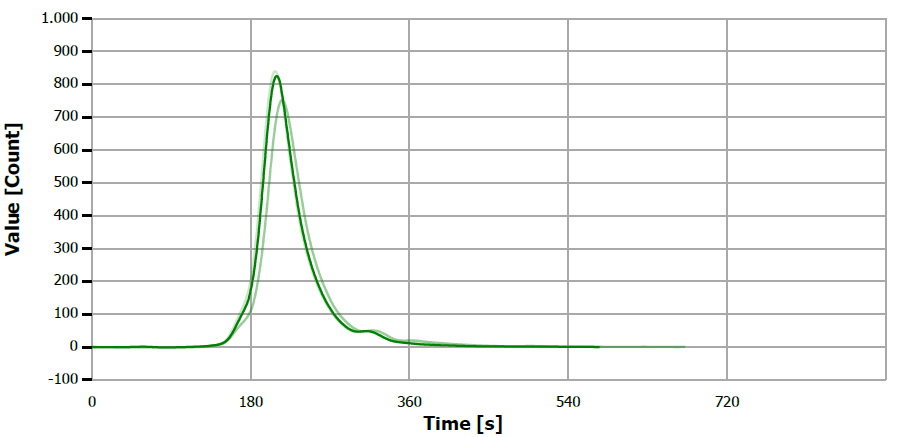
Figure 16. TS measuring curve for standard “5 mg/L S”. Image Credit: Analytik Jena US
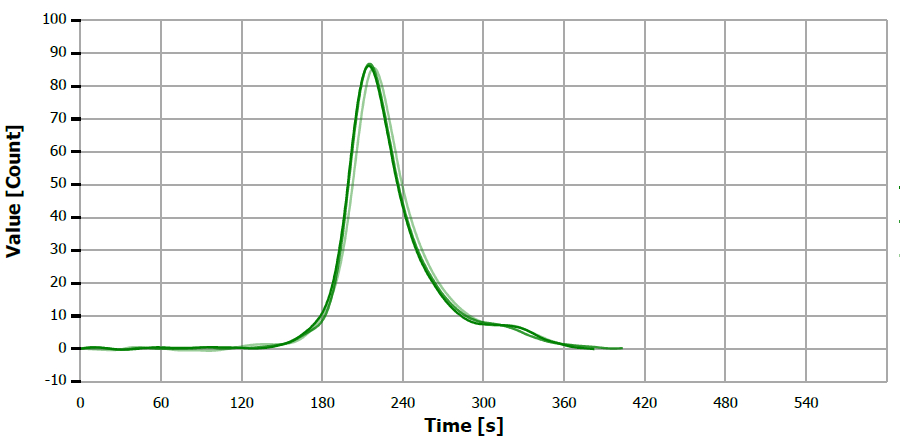
Figure 17. TS measuring curve for standard “0.51 mg/L S”. Image Credit: Analytik Jena US
Summary
Utilizing the multi EA 5100 with the MMS-T autosampler in horizontal mode offers a direct and time-efficient analysis of viscous sample matrices, such as edible oils. Through the effective heating of the sample tray and dosing syringe in this liquid autosampler, dosing viscous matrices becomes feasible without prior dilution steps.
This process saves operational time and expenses while minimizing solvent waste and reducing the potential for operator errors. This functionality is crucial in ensuring trustworthy results, particularly for determining ultra-low element contents near the detection limit.
The flame sensor-assisted, fully automated optimization of the combustion process establishes optimal digestion conditions for any organic matrix. This prevents incomplete combustion and system contamination, eliminating the need for manual adjustments or specific boat programs.
A single method is enough to analyze the full spectrum of liquid matrices, independent of their viscosity, volatility, combustibility, and concentration. This eliminates the necessity for matrix-specific method development and calibration, guaranteeing the best comparability of results.
If required, the application range of the analysis system can easily be extended by accessory modules for the determination of nitrogen, chlorine and/or carbon contents.

Figure 18. multi EA 5100 with ABD and MMS autosampler. Image Credit: Analytik Jena US
Recommended Device Configuration
Table 5. Overview of devices and accessories. Source: Analytik Jena US
| Article |
Article number |
Description |
| multi EA 5100 |
450-300.011 |
multi EA 5100 – combustion elemental analyzer for sulfur, nitrogen, chlorine, and carbon analysis in solids, liquids, gases |
| C/N/S high performance drier kit |
450-300.012 |
High performance reaction gas drier for analysis of carbon, nitrogen and sulfur |
| S module basic |
450-300.021 |
Extension of multi EA 5100 for sulfur determination with UV-fluorescence |
| ABD |
450-300.013 |
Automatic boat drive with flame sensor technology – for horizontal operation of multi EA 5100 |
| MMS-T |
450-900.453 |
Temperature-controlled autosampler – heating /cooling option for liquids, extendable for solids analysis |
| multiWin software |
450-011.803 |
Operation and data evaluation software for multi EA 5100 |

This information has been sourced, reviewed and adapted from materials provided by Analytik Jena US.
For more information on this source, please visit Analytik Jena US.