Mar 19 2015
Scientists at the Fluid Interface Reactions, Structures, and Transport (FIRST) Energy Frontier Research Center (EFRC) have demonstrated that graphene could be used as a proton-selective permeable membrane, which could form the basis for better efficient energy technologies that include improved fuel cells. The FIRST EFRC is led by the US Department of Energy’s (DOE) Oak Ridge National Laboratory (ORNL).
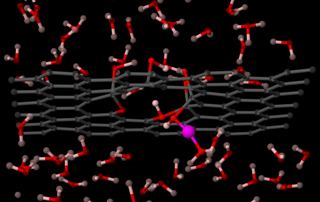 Computer simulations show a single proton (pink) can cross graphene by passing through the world’s thinnest proton channel. Image courtesy of Franz Geiger, Northwestern University
Computer simulations show a single proton (pink) can cross graphene by passing through the world’s thinnest proton channel. Image courtesy of Franz Geiger, Northwestern University
Graphene is a one-atom-thick thick material that has a lightweight and strong carbon honeycombed structure. It is considered as a promising material for energy research and development. This study shows that unprecedented proton movement takes place through inherent gaps or atomic-scale defects in graphene.
“Now you’re able to take a barrier that you can make very thin, like graphene, and change it so you build gates on a molecular scale,” says principal investigator Franz Geiger of Northwestern University, the senior author and a FIRST researcher.
Six years ago this study was initiated as part of DOE’s EFRC’s efforts to speed up scientific breakthroughs necessary for building a new energy economy for the 21st century. FIRST Director David Wesolowski states that, FIRST aims to use interdisciplinary research for developing a fundamental understanding, and also predictive, verified models of the distinct nanoscale environment that exists at interfaces of fluids and solids, and this will facilitate transformative advances in catalysis and electrical energy storage.
This study paper has been authored by 15 FIRST researchers who are from diverse science backgrounds, such as from computer modeling and chemistry. The team contributed their expertise to explore graphene’s structure and mechanisms. They utilized a multifaceted experimental, theoretical, computational and materials synthesis approach for this study.
Pristine graphene was considered to be impenetrable as it was made up of a tight lattice of carbon. This lattice was similar to chicken wire. However, recent research has shown that when graphene was in aqueous solution, it allowed quite a number of protons to go through its atomic structure.
Ivan Vlassiouk of ORNL led the creation of an atomically thin layer of graphene on fused silica. Vlassiouk is an expert in 2D synthesis of materials such as graphene, utilizing chemical vapor deposition techniques.
Raymond Unocic of the ORNL’s Center for Nanophase Materials Sciences then analyzed this graphene utilizing an aberration-corrected scanning transmission electron microscope (STEM). STEM is a high-powered microscope that enables direct imaging of single carbon atoms that are in the adjoining hexagons of graphene. The team observed rare, atomic-scale defects in graphene. These defects allowed aqueous protons to go through the single, thin layer of graphene.
Standard microscopic techniques cannot help detect these regions of missing atoms as they are very small. ORNL’s STEM facility played an important role in this analysis. “To be able to see these images—the individual positions of the carbon atoms in the graphene—is just spectacular,” says Geiger.
The paths of movement followed by the protons were then isolated by the researchers. Then a trap for the “hopping protons” was designed. A single-layer sliver of graphene was created on silica glass. Molecules of water separated these from the glass.
Modifications in the aqueous solution’s acidity on both sides of the graphene layer exposed the gating mechanism that existed in graphene’s structure. The researchers detected this change using the second harmonic generation laser technique.
“The major advantage of second harmonic generation,” says Northwestern’s Jennifer Achtyl, lead author of the Nature Communication article, “is that it is highly sensitive to chemistry at the interface or, in this case, the nanometer-thick environment between the aqueous solution and the surface of the silica. This acute sensitivity and the fact that these experiments can be run nondestructively were critical to our ability to capture experimental evidence of the transfer of protons through graphene.”
The FIRST researchers analyzed the configurations of graphene’s defects using computational methods, and they were able to isolate the occurrences of proton-transfer taking place at the defect areas. They also showed that under normal conditions, even very small molecules, such as of hydrogen and helium could not pass through the proton gates.
“Finally, when we were able to put all the pieces together, we made a conclusive statement that—even though there’s a high energetic barrier for proton transport through graphene—if you lower that energetic barrier, you can allow protons to pass right through,” says Unocic. “This opens a new pathway for the atomic-scale engineering of graphene.”
In this study, the scientists had concentrated on the fundamental mechanics of graphene surfaces. However, the results obtained from this study show that graphene could be developed further for the energy economy.
Fuel cells are one area that holds significant promise. However, significant issues exist, which include fleeting efficiency and cumbersome size. Isolating graphene’s structural gaps and single ion-transfer mechanisms could help advance transportation, production and utilization of energy.
“We’ve looked at this problem from really as many sides as you can possibly look at it with today’s technology,” Geiger says. “It makes a very strong case for taking the effect that we’ve observed and the mechanism that we’ve found and doing something technologically relevant with it. There are so many people working with graphene that to show how aqueous protons actually transfer across graphene will make a big difference.”
The study titled “Aqueous Proton Transfer Across Single Layer Graphene” has been published in Nature Communications.
The coauthors of the study are Unocic, Vlassiouk, Panchapakesan Ganesh, Sheng Dai, Robert Sacci, Pasquale Fulvio, and Wesolowski of ORNL; Geiger and Achtyl from Northwestern University; Yu Cai, Lijun Xu and Matthew Neurock from the University of Virginia, and who are now at the University of Minnesota; and Muralikrishna Raju, Adri van Duin and Weiwei Zhang from the Pennsylvania State University.
The FIRST EFRC Center, which is funded by the US Department of Energy’s Office of Science, has provided support for this study.