Apr 7 2016
In an effort to design novel materials for energy applications, scientists have developed a unique system to make artificial polymers that mimic the ubiquitous proteins, which are nature's own polymers and are involved in all aspects of life.
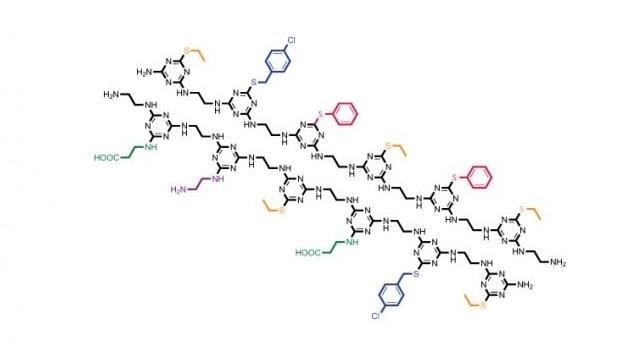 Unique sequences of side chains (in various colors) hang off the TZP-based backbone (black) in this inexpensive, easy-to-synthesize polymer system that mimics proteins in important ways. (CREDIT: Jay Grate/PNNL)
Unique sequences of side chains (in various colors) hang off the TZP-based backbone (black) in this inexpensive, easy-to-synthesize polymer system that mimics proteins in important ways. (CREDIT: Jay Grate/PNNL)
The artificial polymers are based on a low-cost industrial chemical and provides potential to produce materials that exhibit unlimited functions like that of proteins. The study has been published in Angewandte Chemie International Edition
The synthetic polymers produced from the new method imitate proteins in the flexibility of their raw ingredients. This new technique also shows how these ingredients join together to create a structure of a larger size.
Proteins are sequence-defined polymers and have a whole variety of exquisite functions, but natural materials are unstable. That's good for nature, but if we want stable, long-lasting materials, we need to make our own sequence-defined polymers.
Jay Grate, Materials Scientist, Pacific Northwest National Laboratory, Department of Energy
Stuff of life
In living things, proteins function as an engineer and architect and form the very core of life. They are the machines and wrenches that construct different parts of an organism, constructing these parts from different sizes and shapes of proteins. Proteins run the plants, form power plants in cells, make and store energy, and make things grow. They also serve as the bricks of growth. Proteins have become a favorite tool among the research community due to their versatility. A large number of drugs, for example, drugs with names ending in –mab, are re-designed proteins, like converted antibodies. However, proteins have a short life and have been designed to be recyclable and temporary. Proteins find themselves in environments that are full of things - often other proteins - that break them down. This issue can be resolved by developing a material that behaves similar to proteins, but is not a true protein.
Scientists are studying materials that imitate amino acids, which are proteins’ building blocks. Amino acids impart remarkable versatility and variety on proteins. Plastic protein researchers are attempting to imitate these very characteristics. Available in 20 or so variations, each amino acid features the same backbone, from which an atom group - known as side chain - provides a unique chemical property to this amino acid. The backbones in the amino acid break together like beads on a string, with the side chains assembled in a specific order for individual proteins. However, proteins cannot be considered as flaccid pearl necklaces, because the beads fold upon themselves to create structured objects. Different proteins take different shapes; some ending up like olive wreaths, some look like balls, and others like capital Ys. These varied shapes occur as a result of the side chains, and the protein backbone bind to the backbone regions and the other side chains, just like Post-Its stick to each other. The sticking and folding process is similar to an origami process, forming a specific structure instead of a twisted mess.
Threes
Three things were required to imitate proteins. These include; raw components with a backbone to support a wide range of side chains, the stickiness known as non-covalent bonds, and the ability to arrange the side chains in a specific order.
Grate had been studying cyanuric chloride, an industrial chemical for other purposes. He is well aware of the chemical properties of cyanuric chloride and this is what made him initiate the study.
Cyanuric chloride contains three easy places that can be expanded. Of these, two are able to join together, similar to two individuals holding hands, to create the backbone, while the third place can accommodate a side chain. Grate dubbed the resulting molecule a triazine-based polymer (TZP). While this type of polymer would be impervious to protein-destroying entities, Grate believes that environmental things like bacteria will decompose it based on the chemical nature of TZP. So the material would last, just not forever.
With this concept in hand, Grate along with Kai-For Mo, a PNNL chemist, devised a new way to produce TZP. In this method different types of monomers were developed, introducing varied side chains to cyanuric chloride, with an individual monomer acting as a building block similar to an amino acid.
The researchers produced five types of side chains and eventually discovered that one monomer can be added at a time by just altering the temperature where they carried out the chemical reactions, amongst other production tricks.
Once six monomers long, known as 6-mer in polymer parlance, were synthesized, the team validated its creations using analytical systems to reveal that the polymers were the right size and had the right side chains, arranged in the right order. A 12-mer was also developed to demonstrate that this novel technique also works for longer polymers.
In order to observe whether TZPs are able to fold in a similar way to proteins, Michael Daily, a PNNL computer scientist, replicated tiny TZPs, individually and interacted with one another. It was seen that the 6-mer folded in half precisely, creating a straight rod two monomers wide and three monomers long, similar to a hairpin. In a similar way, two 3-mers lined up along one another and partly entwined like a zipper.
The stickiness was non-covalent bonds holding the nanorods between the backbone atoms, the same kinds of bonds used by nature so that proteins assume their correct shapes. Similar to the protein structures, the side chains in the TZP were organized in particular positions over the rods’ exterior. These rods were formed by the interactions between the backbones.
The team is planning to produce a huge library of side chains, and they currently have 10. Longer polymers would then need to be made to show that they also assume their proper shapes. Once the rules are understood on how to achieve certain shapes with TZPs, which also arrange into larger structures, new materials with preferred functions can then be developed, for instance, a catalyst for fuel cells, a membrane for batteries, or a therapeutic drug.