May 23 2016
Plastic manufacturing is a process that consumes significant amounts of energy. Research conducted at the National Institute of Standards and Technology (NIST) has discovered a way to decrease the energy demand in one major step of manufacturing plastic by making use of a group of materials, which can filter impurities more effectively compared to traditional manufacturing process.
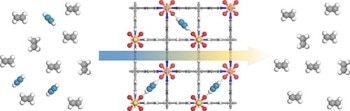 Ethylene (on left in gray) is usually contaminated with acetylene (blue), which can ruin the process that creates the polyethylene used in most plastic. SIFSIX metal-organic frameworks (center) can capture the acetylene efficiently, leaving pure ethylene (right). Credit: Zhou/NIST
Ethylene (on left in gray) is usually contaminated with acetylene (blue), which can ruin the process that creates the polyethylene used in most plastic. SIFSIX metal-organic frameworks (center) can capture the acetylene efficiently, leaving pure ethylene (right). Credit: Zhou/NIST
The results, published in the Science journal illustrate that, materials referred to as metal-organic frameworks (MOFs), can efficiently eliminate acetylene, a major source of contaminant, from ethylene, the material from which a large amount of the world’s plastic is manufactured. The research proposes that filtering out acetylene with the use of MOFs would generate highly pure ethylene required by the industry, while sidestepping the present requirement to convert acetylene to ethylene by an expensive catalytic process.
The chemical name of the plastic seen daily—right from grocery bags and water bottles to household gadgets—is polyethylene, a flexible material produced by stringing together long chains of ethylene which is a simpler molecule. The demand for plastic worldwide makes ethylene the most commonly manufactured organic compound, with more than 100 million tons of ethylene produced every year, mostly by crude oil refining.
Recently manufactured ethylene is not pure enough to turn into plastic as the refinement process produces a considerable quantity of acetylene also, which could damage the catalysts that make ethylene molecules to string together. The conventional industrial key is to convert this unwanted acetylene also into ethylene, however this step requires the use of palladium - a costly and rare metal - to be used as a catalyst and also uses a considerable amount of energy.
The research group, which includes researchers from the NIST Center for Neutron Research (NCNR) and five universities from across the globe, discovered that SIFSIX, a family of MOF materials, found in the 1990s, may offer a better choice to remove acetylene. MOFs are porous crystals which when viewed under a microscope look similar to a building under construction - numerous girders with gap in between. The SIFSIX groups name comes from a few of its girders, which are produced from silicon (Si) and six atoms of fluorine (F6).
The group discovered that when ethylene was passed through the MOFs, the fluorine attracted and captured nearly all of the contaminant acetylene, allowing the now-purified ethylene to pass without any hindrance. Changing the pore size by varying the length of the girders allowed the MOFs to filter ethylene-containing acetylene in concentrations between 1 and 50%, which are characteristic in industry.
The SIFSIX MOFs have achieved records amongst adsorbent materials for adsorption capacity and selectivity (the capability to only attract acetylene while permitting ethylene to pass). In accordance to the research group, the outcomes prove that the SIFSIX group offers a feasible substitute to conventional industrial practice.
They reduced the amount of acetylene in ethylene down to less than 2 parts per million (ppm), which is lower than the 5 ppm that polyethylene manufacturing requires. SIFSIX MOFs are easy to produce, safe to use, and can be reused over and over again. They also have the advantage of being stable, which is not true of all MOFs.
Wei Zhou, Materials Scientist, NIST
Scientists based at China’s Zhejiang University (by Huabin Xing), Ireland’s University of Limerick, (Michael Zaworotko) and the University of Texas–San Antonio in the U.S developed and investigated the MOFs in detail. UT-San Antonio Professor Banglin Chen sensed the importance of SIFSIX MOFs for this purpose, and arranged and led the group. The NIST’s part of the work, which was concerned with neutron diffraction experiments and computer modeling of MOFs, verified the mechanism by which acetylene was captured by the SIFSIX MOFs. There was also contribution from researchers of the Saudi Arabia’s King Abdullah University of Science and Technology and Netherlands’ University of Amsterdam.