Nov 21 2016
Gold nanoparticle-based supraspheres, which are analogues of supramolecular cages and containers, can be used to perform classical host-guest chemistry, according to new experiments by researchers in Israel and Switzerland. Although real-world applications may be a way off, at the laboratory level these materials may be used as soluble systems for better understanding phenomena associated with the uptake of substrates by porous solid-state materials. Areas such as catalysis, sensing, environmental clean-ups (at small scales and selectively for specific targets) and medicine (drug or diagnostic cargo delivery) might also benefit.
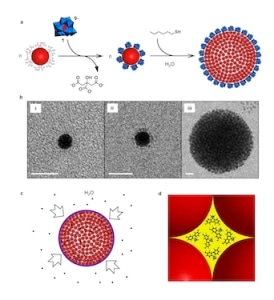 Making colloidal supraspheres. Courtesy: I Weinstock
Making colloidal supraspheres. Courtesy: I Weinstock
“Our work shows that 3D frameworks made up of gold-nanoparticle structural building units can function as nanoscale host assemblies for molecular guests, achieving, at the nanoscale, something that until now was largely limited to either supramolecular chemistry (cages and containers) or solid-state chemistry (zeolites and metal-organic frameworks, or MOFs, for example),” explains team leader Ira Weinstock of Ben-Gurion University of the Negev in Israel.
“Our new gold nanoparticle supraspheres can take up several orders of magnitude more guest molecules than individual supramolecular containers and cages and, what is more, on a gram-per-volume basis (grams of guest molecules per volume of the self-assembled host), the supraspheres can take up as much as MOFs.”
Exploiting organic ligand shells on the gold nanoparticles
“The key to this success is a type of chemoselectivity unique to nanoscale and metal nanoparticles,” he says. “This allows us to use organic ligand shells on the gold nanoparticles at the interface with the external environment (in our case, water) to kinetically control the rate of uptake of specific guests. This control comes thanks to the chemical composition and properties of the ligand shells and guests. Such a phenomenon is very different to that of molecular cages and MOFs, which selectively is most often controlled by pore size and rarely by ligands at the pores.”
The new supraspheres can be thought of as hydrophobic assemblies of 6 nm diameter hydrocarbon coated balls, where the balls are gold nanoparticles protected with alkanethiolate, he adds. “The gold cores are templates for binding organic ligand shells and it is these shells that are responsible for the hydrophobically-driven self-assembly of the particles in water and the hydrophobically-driven uptake of organic guests from water.
“Once assembled, the hydrocarbon-coated nanoparticles form a close-packed structure, and as in all such structures, the interior is made up of a percolated network of void spaces (N octahedral “holes” and 2N tetrahedral “holes”, where N is the number of gold nanoparticles in the suprasphere)," he explains. “Since the gold nanoparticles are coated with hydrocarbon shells, these holes possess hydrophobic walls and are thus hydrophobic cavities.”
Quantifying the number of guest molecules taken up
To quantify the number of guest molecules taken up by each suprasphere, the researchers used two techniques. “We first measured the uptake of the chemicals bisphenol A and azulene using spectroscopy,” says Weinstock. “We observed these chemicals as very dilute species in water by using UV-vis spectroscopy for bisphenols A and fluorescence spectroscopy in the visible region for azulene. We incrementally added specific concentrations of the supraspheres, and after each addition determined the concentration of guests remaining in the water.
“The second method involves not only uptake and quantification but also transport and release,” he says. “Here, we add the supraspheres to dilute aqueous solutions of the hydrophobic guests and allowed them to reach equilibrium. We then isolated the supraspheres by centrifugation, separated them as a solid pellet and then dissolved them in deuteromethylene chloride. Here, the hyrdrophobic driving forces no longer exist so the supraspheres dissolve into individual alkanethiolate-capped nanoparticles, releasing their cargos of hydrophobic substrates. We then quantify these using H-1 nuclear magnetic spectroscopy (NMR).”
The team, which includes researchers from the Ecole Polytechnique Fédérale de Lausanne in Switzerland, says it will now try to decrease the size of the constituent gold nanoparticles to make the suprasphere pores smaller. “We would also like to better understand the chemical selectivity we discovered during the course of this research and so develop clear relationships between ligand-shell composition, and structure and selectivity for specific guest molecules and functional groups,” says Weinstock.
“A final application mentioned briefly in our work, published in Nature Nanotechnology doi:10.1038/nnano.2016.233, is to use the optical cavity of the gold-nanoparticle-based supraspheres to sequester and protect light-sensitive guests.”