Apr 28 2017
Tiny crystals twist, align and slam into each other, like two magnets being pulled toward each other, because of an altogether different force. For the very first time, researchers have measured the force that pulls these crystals together and visualized how they swivel and align.
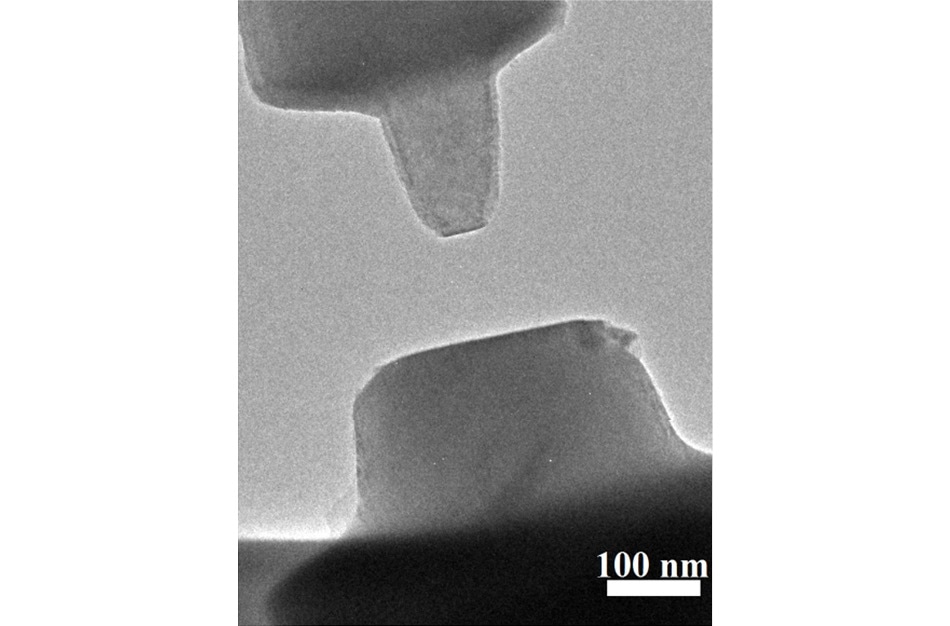 Tiny, milled pieces of a titanium oxide mineral called rutile -- top left, bottom right -- face off within a high resolution microscope enhanced with the ability to measure minuscule forces called van der Waals forces. Credit Xin Zhang et al
Tiny, milled pieces of a titanium oxide mineral called rutile -- top left, bottom right -- face off within a high resolution microscope enhanced with the ability to measure minuscule forces called van der Waals forces. Credit Xin Zhang et al
Referred to as van der Waals forces, the attraction offers insights into how crystals self-assemble. This is regarded as an activity that takes place in a wide range of cases in nature, from bones to shells to rocks.
It's provocative in the sense that from these kinds of measurements one can build a model of 3-D assembly, with particles attaching to each other in select ways like Lego bricks. Crystals are most everywhere in nature, and this work will help us take advantage of these forces when we design new materials.
Chemist Kevin Rosso of the Department of Energy's Pacific Northwest National Laboratory.
Fusion force
Crystals develop supporting structures in a wide variety of synthetic and natural materials. It is possible for larger crystals to build up from smaller ones. Crystals are generally shaped like cubes and yet have several different sides, some of which do not match at all and the others indeed match well with each other. Crystals can fuse seamlessly, growing bigger and bigger, when matching sides are properly oriented.
The questions that come up at this point focus on what makes crystals get close enough to fuse in the first place, and can they actually self-align. Even though a number of forces have been hinted at for a very long time, the tools that will help to narrow down the correct ones are yet to be discovered.
Rosso and teams at PNNL, EMSL, the Environmental Molecular Sciences Laboratory, a DOE Office of Science User Facility at PNNL, and the University of Pittsburgh recently developed a new approach by incorporating an environmental transmission electron microscope (ETEM) with nanocrystal force probes that permits scientists to watch crystals interact in a life-like situation. PNNL post-doctoral chemist Xin Zhang and EMSL user Yang He, a Ph.D. student from the University of Pittsburgh, analyzed how titanium oxide crystals couple by using resources within EMSL.
Their experiment can be understood by imagining two magnets being brought closer to each other. These magnets will jump together when they are very close such that the attractive force overcomes the effort being used to hold them away from each other. This was performed by the PNNL team on a much smaller scale and with a force that is not magnetism.
One small jump
The team had to use extremely small crystals that would not overwhelm the weak forces they expected to see. The researchers attached titanium oxide crystals a hundred to a thousand times thinner than a strand of human hair (based on the hair) to either side of an instrument that measures force. The crystals were then moved toward each other, twisted at varied angles between them, until both the crystals snapped together.
The researchers also pulled the crystals apart and then measured the amount of force used for carrying out that task. With these measurements, the researchers were able to characterize the force in detail. Different forces are available that work for objects of this size, and with the extra analyses the researchers concluded that van der Waals forces were the ones at work causing self-alignment.
Pulling Apart Titanium Oxide Surfaces
Researchers slowly pull apart tiny bits of a titanium oxide mineral called rutile, about a thousand times skinnier than a human hair. The red dots near the top show the starting point. As the bottom half is pulled down, the top half stays attached by van der Waals forces -- until, that is, the pull becomes too great.
And a twist
The researchers also wanted to put a face to a name, in a manner of speaking, of a theoretical prediction of van der Waals forces made in the 1970s. With this theory, scientist could calculate the torque between crystals that are being twisted relative to each other (imagine twisting a baguette in order to pull a piece of bread off) based on the angle between them.
The researchers also measured the force existing between two crystals held at a constant distance apart but twisted in opposite directions from each other. Maria Sushko, co-author and computational physicist, compared the data to predictions the theory made and proved that the theory held up.
This is the first measure and proof that the force depends on how the crystals are rotated relative to each other, what we call rotationally dependent. If they are rotationally dependent, this implies that this force will contribute to aligning free crystals that bump together in a liquid environment, for example, increasing the rate of successful sticking.
Rosso
Additionally, establishing the connection highlights the fact that it will be easier to determine such attractive forces for crystals developed from different materials, such as calcium carbonate present in seashells. By just plugging in numbers to an equation instead of re-doing all of the experiments, scientists will be able to determine these forces.
The Department of Energy Office of Science and PNNL Laboratory Directed Research and Development program supported this work. The experimental work was performed at EMSL, one of a handful of DOE Office of Science User Facilities equipped with the scientific expertise and technology to conduct this research.