Jun 22 2017
It is presently essential to design new catalysts for developing new and useful organosilicon compounds, which are in great demand in fields ranging from the electronics to the medical industries.
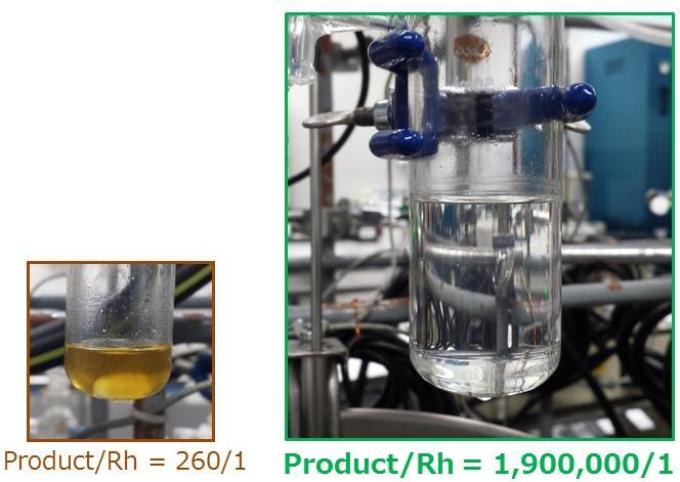 This is a view of the hydrosilylation reaction using the SiO2-supported catalyst consisting of an immobilised Rh complex and tertiary amines, with a turnover of 260 (left) and approaching 1,900,000 (right). The very low loading of Rh was well presented by the clear solution (right). Credit: Tokyo Institute of Technology
This is a view of the hydrosilylation reaction using the SiO2-supported catalyst consisting of an immobilised Rh complex and tertiary amines, with a turnover of 260 (left) and approaching 1,900,000 (right). The very low loading of Rh was well presented by the clear solution (right). Credit: Tokyo Institute of Technology
Hydrosilylation, referring to the formation of carbon-silicon bonds, is considered to be a vital step in this process. Additionally, an increased amount of interest has been shifted on rhodium-based catalysts known to play an efficient role in accelerating this reaction.
Ken Motokura of Tokyo Institute of technology (Tokyo Tech) and colleagues have now devised a new catalyst made up of three core components (a rhodium (Rh) complex and a tertiary amine (NEt2) on silica (SiO2)) that brings about a major improvement in the hydrosilylation process.
A report featured in ACS Catalysis highlights that the new catalyst obtained a turnover number of approximately 1,900,000 over a time span of 24 hours, far surpassing other supported-rhodium catalysts that have been developed to date. The co-immobilized amine (NEt2) is considered to be a major factor behind the enhanced catalytic activity.
Although the specific reason for improvement is still unclear, we know that usually the hydrosilylation reaction is accelerated by electron donation to the rhodium center, and the tertiary amine has electron-donating ability.
Ken Motokura, Tokyo Institute of Technology
According to the new study, having both the Rh complex and amine on the SiO2 surface will help generate a higher yield (96%) than with only Rh (9%) or just amine (less than 1%), thus signifying a synergistic effect at play.
Notably, the catalytic performance was affected by the order in which the Rh complex and amine were immobilized. Motokura explains that the immobilization timing could affect the positioning of the Rh complex and amine, which eventually affects catalytic activity. This finding concurs with an earlier study conducted by the same team, which discovered that catalytic activity firmly relied on the proximity of the Rh complex and tertiary amine.
The high cost of rhodium is one limiting factor for future studies. "In this study, it's important to note that we were able to achieve very low loading of rhodium," says Motokura. "We recognise that finding alternatives to rhodium will be critical. So far, however, catalysts based on inexpensive metals generally show low activity."
The team next focuses on using non-precious metal and organic functions on the same surface to produce a synergistic effect in order to obtain catalytic performance on the same level with rhodium-based catalysts.
If this succeeds, our long-held goal of developing sustainable solutions based on chemistry will be realized.
Ken Motokura, Tokyo Institute of Technology