Jun 20 2019
A research team from Nanchang University has tried to directly construct the surface structure of copper (Cu)-based substrate to obtain a sequence of Ce-O-Cu catalysts for NH3-SCR of NO. The study will soon be published in the upcoming issue in NANO.
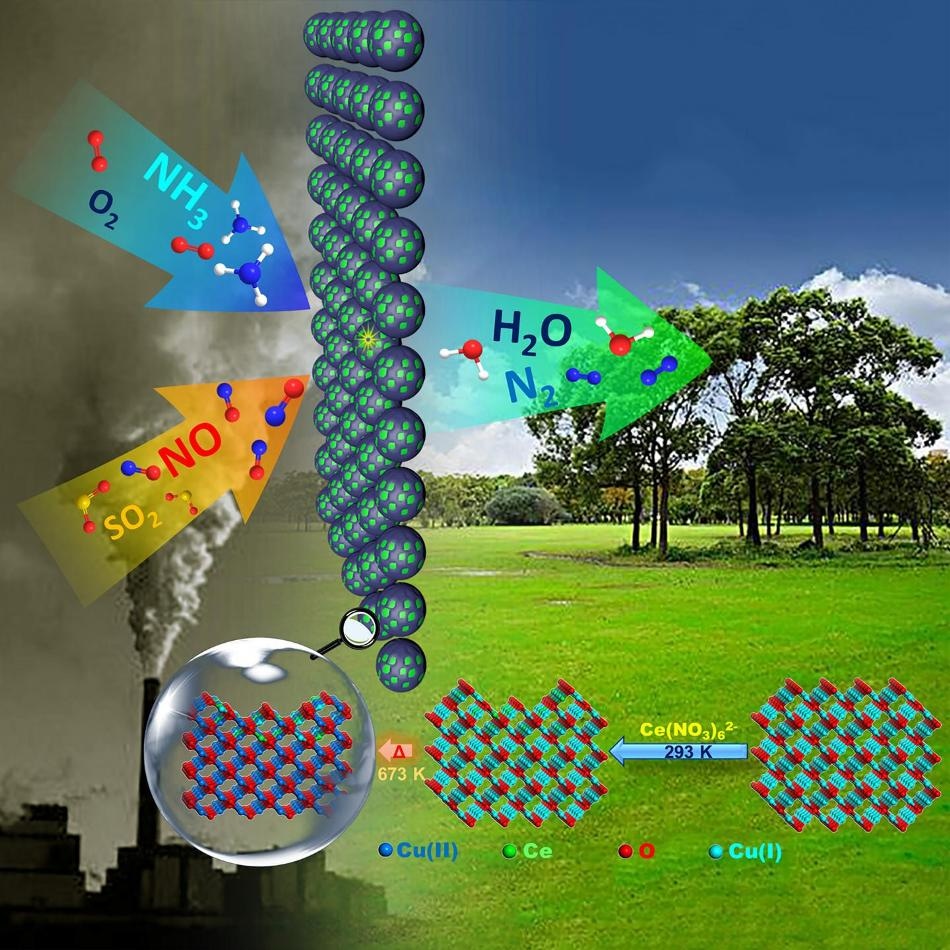 Chemically weaving the surface of Cu-based oxide with Ce initiated from employing Ce(IV) precursor to etch-embed Ce into Cu2O surface was demonstrated to be quite efficient for construction of synergistic catalysis interface in nano or sub-nanoscale, leading to the dramatic activity enhancements for NH3-SCR of NO, especially when SO2 is present. (Image credit: Dan Zhao et al.)
Chemically weaving the surface of Cu-based oxide with Ce initiated from employing Ce(IV) precursor to etch-embed Ce into Cu2O surface was demonstrated to be quite efficient for construction of synergistic catalysis interface in nano or sub-nanoscale, leading to the dramatic activity enhancements for NH3-SCR of NO, especially when SO2 is present. (Image credit: Dan Zhao et al.)
The resultant catalysts were structured as CuO matrix with an interactive surface made up of Ce(III)-Ce(IV) and Cu(I)-Cu(II) co-present species, displaying the clear synergistic effect and resulting in striking catalytic performance even with the presence of SO2 in the reactant mixture.
So, what is the viable preparation method for designing oxide composites that have interactive structures for sophisticated heterogeneous catalysis? An attempt was made here to directly engineer and control the Ce-O-Cu composited surface structures from the Ce(IV) design to chemically integrate the Ce into the Cu2O substrate surface, and moreover, the samples that were derived thermally were also examined as catalysts for NH3 selective catalytic reduction (NH3-SCR) of NO.
The characteristic of the surface structure of a solid catalyst, like oxide composites, was the crucial factor to control the performance for heterogeneous catalysis reactions. Conversely, the conventional preparation techniques to build oxide composites, like solvothermal synthesis, sol-gel, or co-precipitation, were not appropriate to exploit the solid materials’ surface structure.
In this analysis, contrary to these body-mingling preparations from conventional techniques, an attempt was made to directly build and control a composited oxide surface on a redox replacement preparation by using Ce(IV) precursor to etch Cu2O. This way, Ce species can be embedded into copper-based oxide surface, and the catalysts obtained through thermal stabilization treatments were examined for NH3-SCR of NO.
Theoretically, 0.20 V and 1.72 V were the standard potentials for two redox pairs [Cu2O/Cu2+] and [Ce(IV)/Ce(III)], respectively; for [Cu2O/Cu2+] pair, the lower potential implies that Cu2O would serve as the reductant when it comes across the Ce(IV) species, that is, Ce(IV) species can possibly etch Cu2O to create Ce(III) and Cu2+ species.
Given that Cu2+ would be removed in an aqueous solution, the left space on a copper-based solid substrate may enable the Ce species to be embedded into the surface. While Cu(II) contained compounds like CuO that were invariably utilized as substrates or precursors, such types of Cu(II) compounds will not be able to react with Ce ion species, regardless of whether Ce(III) or Ce(IV) was used owing to the thermodynamic restriction from the above redox potential difference.
Hence, the preparation and design of Ce-O-Cu composited samples started from using Ce(IV) contained Ce(NO3)62– ion so as to etch-embed the Ce onto the surface of the Cu2O substrate, and this was followed by thermal-stabilization processing in order to obtain catalysts.
With complete characterizations, the design was shown to be relatively efficient in producing Ce(III)-Ce(IV) and Cu(I)-Cu(II) co-present interactive surface on CuO matrix with an extremely low dosage of Ce (0.83-2.3 wt.%) when compared to literature works. More fascinatingly, the surface reactivity, surface metal species distributions, strength, active site distributions, and redox properties could be easily and sensitively controlled on such kinds of structures, which provide an option to obtain better catalytic performance like fold-increased TOF, higher NO conversion (≥90% with ≥95% selectivity to N2) below 300 °C, and excellent catalytic durability despite the presence of SO2 on well-controlled sample when compared to Ce-Cu reference catalysts prepared from the conventional technique.
All the above catalytic outcomes were achieved with gas hourly space velocity, or GHSV, at 100,000 h–1, and also, more viable reaction conditions like (SO2+H2O) co-presence were imposed on the samples. The excellent performance under these conditions along with new structure and the samples’ appealing physical chemistry characteristics indicate that derived structures and chemically embedding preparation could provide an economical solution to achieve efficient oxide composite materials for both advanced and feasible applications.
The authors thankfully acknowledge the financial support provided by the National Natural Science Foundation of China (NSFC, No.21003071, No.21563018 and No.21663016) and Doctoral Fund of Ministry of Education of China (No.20093601120007).
Other authors are Qian Sun, Chun Zeng, Meng-Meng Xing, Bo Chen, San-Guo Hong (Professor) and Ning Zhang (Professor), Qian Sun, and Chun Zeng who contributed equally to the paper; Dan Zhao (Associate Professor) is the corresponding author of the paper.
Source: https://www.worldscientific.com/