Many chemical processes require the use of a catalyst. It allows a molecule to transform into another without changing itself, allowing it to be reused several times. Many catalysts, on the other hand, are constructed of precious metals, making them costly and possibly hazardous to the environment.
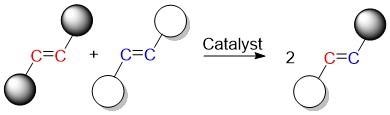 The olefin metathesis reaction produces new carbon-carbon double bonds by breaking the original double bonds and regenerating new ones. Image Credit: Okinawa Institute of Science and Technology Graduate University.
The olefin metathesis reaction produces new carbon-carbon double bonds by breaking the original double bonds and regenerating new ones. Image Credit: Okinawa Institute of Science and Technology Graduate University.
Researchers have now developed a catalyst made of iron, a much more abundant element, to speed up an important chemical reaction known as olefin metathesis. Their findings were just reported in Nature Catalysis.
The olefin metathesis reaction is among the most widely applicable catalytic reactions for carbon-carbon double bond formation. Carbon-carbon double bonds are an important bond found in many chemical products.
Satoshi Takebayashi, Researcher, Okinawa Institute of Science and Technology Graduate University
Olefins are a type of carbon-carbon double bonding chemical. By switching the carbon atoms in olefins, the olefin metathesis reaction creates new carbon-carbon double bonds. By disrupting the initial double bonds and forcing new ones to form, the catalyst enables this swapping.
The valuable metal ruthenium is currently one of the most preferred catalysts for this reaction. The goal of this research was to speed up the reaction by employing a catalyst made of iron, a far more abundant metal, making the entire process less expensive and more environmentally benign. Since ruthenium and iron are in the same group on the periodic table and are believed to have similar characteristics, this has been a long-sought objective in the science establishment.
The researchers created a novel iron complex and showed that it could be utilized as a catalyst in the olefin metathesis reaction in this work. They demonstrated it by making a polymer, which is a long-chain molecule made up of many smaller chemical units.
Considering the success of this study, Takebayashi pointed out that current ruthenium-based catalysts are still far more useful than newly developed iron-based catalysts. When exposed to air and moisture, the iron catalyst becomes unstable and less active. Before the iron catalyst can replace the ruthenium catalyst, these constraints must be overcome.
This study can be useful to other researchers in the field. I hope that iron-based catalysts can be developed further using this knowledge.
Satoshi Takebayashi, Researcher, Okinawa Institute of Science and Technology Graduate University
Journal Reference:
Takebayashi, S., et al. (2022) Iron-catalyzed ring-opening metathesis polymerization of olefins and mechanistic studies. Nature Catalysis. doi.org/10.1038/s41929-022-00793-4.