 By Surbhi JainOct 18 2022Reviewed by Susha Cheriyedath, M.Sc.
By Surbhi JainOct 18 2022Reviewed by Susha Cheriyedath, M.Sc.In an article recently published in the open-access journal Scientific Reports, researchers discussed the removal of lead (II) ions through the synthesis of powdered and beaded chitosan materials treated with ZnO.
Study: Synthesis of powdered and beaded chitosan materials modified with ZnO for removing lead (II) ions. Image Credit: Raimundo79/Shutterstock.com
Background
Lead may flow into wastewater because it is used as a raw ingredient in many industries. It is one of the most dangerous elements, and due to its toxicity, it can cause major human systems to malfunction. Therefore, it is strongly advised to reduce the amount of lead contamination in wastewater for a safe environment, including human health.
Lead removal from wastewater can be accomplished using a variety of techniques, but each technique has benefits and drawbacks that must be considered before usage. The operating and disposal expenses are the main deciding considerations, and a reasonable cost with a practical solution is recommended. Applying an adsorption approach to remove lead from wastewater is an intriguing technique.
An alternate strategy for lowering food waste and utilizing the advantages of chitosan as heavy metal adsorbents is to use animals with shells and carapaces as the raw materials for chitosan production. Chitosan can remove different heavy metals from wastewater. Various metal oxides have been employed in numerous studies to enhance heavy metal adsorbents. However, due to issues with pressure drop, clogging, and difficult separation after treatments, it may be a good idea to incorporate metal oxide into an effective adsorbent to encourage a high adsorbent efficiency for removing a particular target pollutant. The creation of a long-lasting adsorbent form may also aid in long-term use and reduce operating expenses.

Physical characteristics of (a) chitosan standard, (b) chitosan powder (CP), (c) chitosan beads (CB), (d) chitosan beads mixed ZnO (CZB), and (e) chitosan beads coated ZnO (ZCB). Image Credit: Ngamsurach, P et al., Scientific Reports
About the Study
In this study, the authors discussed the development and characterization of chitosan materials that were treated with ZnO using a variety of procedures. Adsorption isotherms, batch tests, and kinetics were used to investigate the lead removal effectiveness of chitosan materials. Chitosan powder (CP), chitosan beads combined with ZnO (CZB), chitosan beads (CB), and chitosan beads coated with ZnO (ZCB) were created. While CB was an amorphous form, CP indicated a semi-crystalline structure. Semi-crystalline structures with ZnO peaks were CZB and ZCB.
While CB, CZB, and ZCB were sphere forms with coarse and scaly surfaces, respectively, CP had a scaly sheet and coarse texture. The primary chemical components of chitosan materials were C, O, and N, and Zn was found in CZB and ZCB. Chitosan materials' three primary functional groups were O-H, N-H, and C-O.
The team demonstrated that all chitosan-based compounds had high lead removal efficiencies of over 92%, and Freundlich and pseudo-second-order kinetic models explained the adsorption patterns and mechanisms for these materials. It was observed that metal oxide should be added, the form of the material should be changed to increase material efficiency, and ZCB was a solid option for more industrial applications.
The researchers created four chitosan materials. The goal of this study was to remove lead from synthetic wastewater using these materials. By using the Brunauer-Emmett-Teller (BET), field emission scanning electron microscopy focus ion beam (FESEM-FIB), X-ray diffractometer (XRD), Fourier transform infrared spectroscopy (FTIR), and energy dispersive X-ray spectrometer (EDX) techniques, researchers determined the size of their surface area, pore size, pore volume, and crystalline formation.
Additionally, a number of batch tests were carried out to assess the effectiveness of their lead removal, and adsorption isotherms and kinetics were studied to comprehend the patterns and mechanisms of their adsorption.
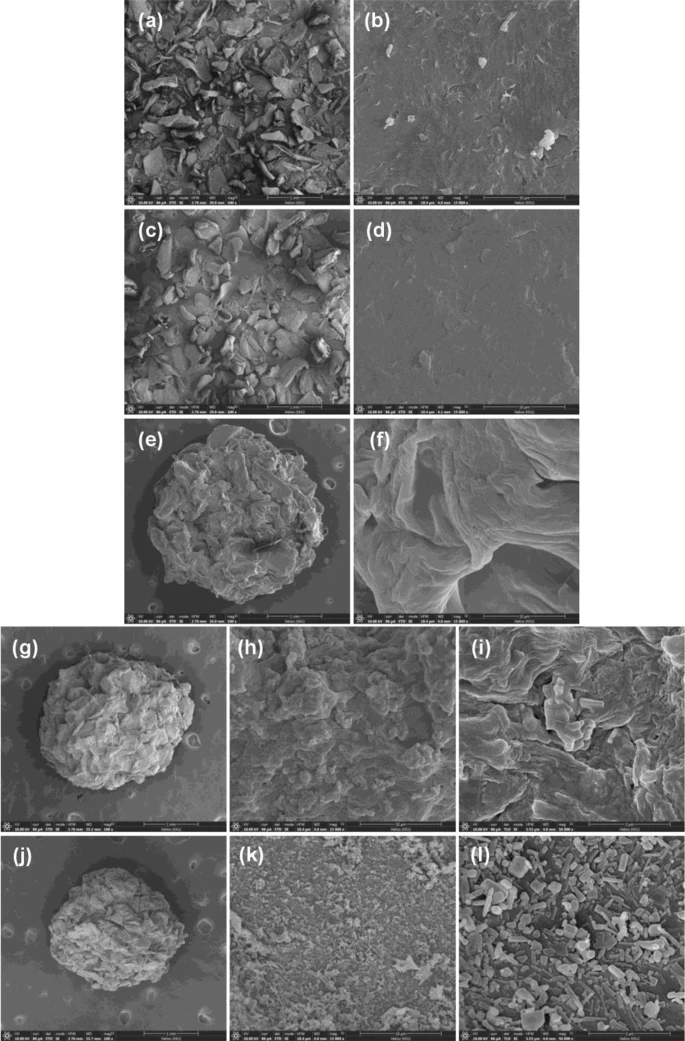
FESEM-FIB images of surface morphology of (a, b) chitosan standard, (c, d) chitosan powder (CP), (e, f) chitosan beads (CB), (g, h, i) chitosan beads mixed ZnO (CZB), and (j, k, l) chitosan beads coated ZnO (ZCB). Image Credit: Ngamsurach, P et al., Scientific Reports
Observations
CP resembled the chitosan standard in color and was a fine yellow powder. CZB and ZCB were light yellow beaded colors, while CB was a yellow beaded hue. ZCB outperformed other chitosan compounds in terms of surface area and pore size. The amorphous structure was represented by CB and the semi-crystalline structure by CP. Semi-crystalline structures were observed for CZB and ZCB that displayed distinctive ZnO peaks. While CB, CZB, and ZCB were sphere forms with scaly-sheet surfaces, CP had a coarse, scaly surface. Additionally, ZnO was found in CZB and ZCB.
All chitosan compounds had the three principal chemical components C, O, and N, whereas Na, Ca, and Cl were also present in CB, CZB, and ZCB. The Zn concentration of CZB and ZCB was also very high. O-H, C-O, N-H, and -COOH, which corresponded to sodium alginate in chitosan beads, were the primary functional groups of chitosan materials. Additionally, ZnO was found in CZB and ZCB and reacted with -OH or NH2 in the chitosan.
The ideal lead adsorption conditions for CP, CB, CZB, and ZCB were 0.5 g, 14 h, pH 5, 0.4 g, 12 h, pH 5, and 0.3 g, 12 h, pH 5, respectively. ZCB had the highest lead removal effectiveness of 98.91% among all chitosan-based compounds, which could all remove lead by more than 92%. Therefore, improving chitosan material efficiencies required both adding ZnO and modifying the material shape.
The pseudo-second-order kinetic models and Freundlich model linked to a physiochemical adsorption process were applicable to all chitosan compounds. Because of its high effectiveness and lack of need for a separate section after treatment, ZCB was the greatest option for industrial applications of wastewater treatment systems while all chitosan materials were potential materials for the adsorption of lead in aqueous solution.
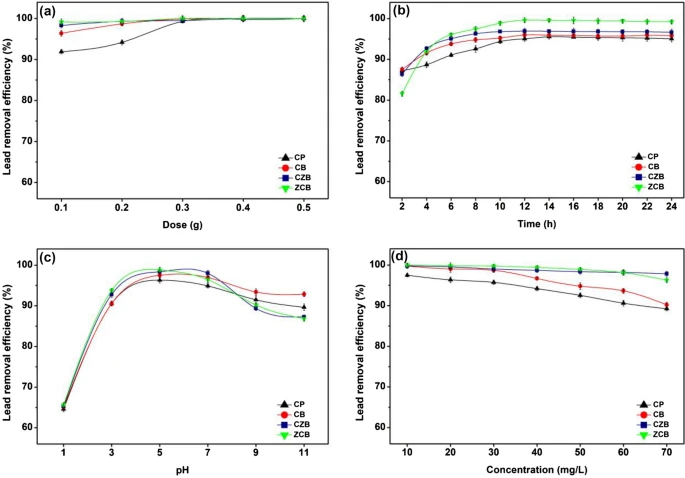
The batch experiments of chitosan powder (CP), chitosan beads (CB), chitosan beads mixed ZnO (CZB), and chitosan beads coated ZnO (ZCB) in (a) dose, (b) contact time, (c) pH, (d) concentration for lead adsorptions. Image Credit: Ngamsurach, P et al., Scientific Reports
Conclusions
In conclusion, this study discussed the development of four chitosan materials—chitosan powder (CP), chitosan beads mixed with ZnO (CZB), chitosan beads (CB), and chitosan beads coated with ZnO (ZCB) for lead adsorptions in an aqueous solution. To increase the effectiveness of chitosan materials for lead removal from wastewater for future industrial applications, this study tried to synthesize chitosan materials by adding metal oxide and modifying the material shape.
The authors mentioned that for subsequent projects, desorption experiments are necessary to investigate the potential for material reuse, and column run tests are also necessary to investigate the potential for industrial applications in continuous flow systems.
Reference
Ngamsurach, P., Namwongsa, N., Praipipat, P., et al. (2022) Synthesis of powdered and beaded chitosan materials modified with ZnO for removing lead (II) ions. Scientific Reports, 12, p. 17184. https://www.nature.com/articles/s41598-022-22182-4
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.