Elemental analyzers are suitable for quality control of an extensive range of products. A wide selection of matrices, such as ceramics, steel, coal or soil, can be examined for their element concentrations with varied types of instruments. The product range of ELTRA GmbH, based near Düsseldorf, Germany, comprises analyzers for C, H, N, O, S and thermogravimetry which permit the standard-compliant determination of carbon in different chemical bondings, as well as nitrogen and oxygen in SiC and in materials containing SiC. The demands that have to be fulfilled for a standard-compliant analysis can, however, differ greatly, based on the parameters to be determined.
Silicon carbide has a high melting point of 2,700 °C and is thus considered to be an essential raw material for ceramic and refractory products. Another characteristic of SiC refers to its resistance against strong acids and chlorine, also at high temperatures. Thanks to a hardness of 9.6 Mohs, it is also employed in the metallurgical industry for the production of polish and abrasives.
The European standard series EN ISO 21068 (2008) regulates the chemical analysis of silicon carbide and raw materials made up of silicon carbide. Part 1 focuses on sampling, part 2 on the chemical analysis of silicon, carbon and loss of ignition and part 3 covers metal analysis and determination of nitrogen and oxygen concentrations.
ELTRA combustion analyzers are best suited for the quality control of ceramic and refractory products which contain silicon carbide. This article outlines the limits and possibilities of elemental analyzers when dealing with these materials.
Thermogravimetric Parameters
The determination of thermogravimetric parameters such as, for instance, loss of ignition, is described in the second part of ISO 21068. Thermogravimetry is based on the continuous recording of mass changes as a function of a combination of time, atmosphere and temperature. The standard clearly defines the application of muffle furnaces and balances for this process. All methods described in the standard employ a defined sample container made of ceramic material, steel or platinum which is pre-heated at the prescribed temperature between 250 °C and 1050 °C. The sample weight is not always defined (example, loss on drying LOD250) or ranges from 2 – 5 g to 1 kg (change of mass in air at 200 °C and 400 °C). After weighing the sample and applying the defined temperature program (example, for LOI850, heating up to 850 °C and maintaining for 3 h), the hot crucibles will have to cool down in the desiccator and are then weighed.
Thermogravimetric analyzers equipped with a combination of furnace and balance significantly simplify the manual procedure. These analyzers usually have an interior chamber which can be heated up to 1,000 °C and a separate weighing cell in the analysis chamber which is attached to the furnace by a ceramic pedestal. A rotating carousel places up to 19 different samples, one after the other, on the pedestal to be weighed. The market offers thermogravimetric analyzers for both small sample quantities (example, 20 mg) and quantities of 5 g or more (such as ELTRA’s Thermostep, Figure. 1). By employing an empty reference crucible, thermal buoyancy is compensated and measurements can be performed reliably even at high temperatures. The ceramic crucibles generally have a volume of 12 ml, permitting sample weights of up to 5 g. This, indeed, is not extremely practical because of the high filling level. So far, the DIN EN ISO 21068-2 standard does not mention automated thermogravimetric analysis.

Figure 1. ELTRA’s thermogravimetric analyzer Thermostep
If a thermogravimetric analyzer is employed for the determination of LOI850 (loss on ignition at 850 °C), it is possible to meet the specifications of the standard to a high degree, if not 100 %.
For a series of tests, the standard Euronorm CRM No. 781-1 was weighed into crucibles which had been preheated to 850 °C in ELTRA’s Thermostep. Figure 2 presents a typical measurement curve and Table 1 contains the equivalent results. The standard was initially dried at 105 °C over night and then three crucibles were filled with 1 g each. The LOI850 value measured with the Thermostep correlates well with the value of free carbon stipulated in the standard.
Table 1. Weight loss of standard CRM 781-1 after heating up to 850 °C/3 h
| Sample no. |
Weight loss / % by mass |
For comparison: Free carbon / % by mass (informative value) |
| 1 |
36.9 |
37.22 |
| 2 |
37.1 |
37.22 |
| 3 |
37.2 |
37.22 |
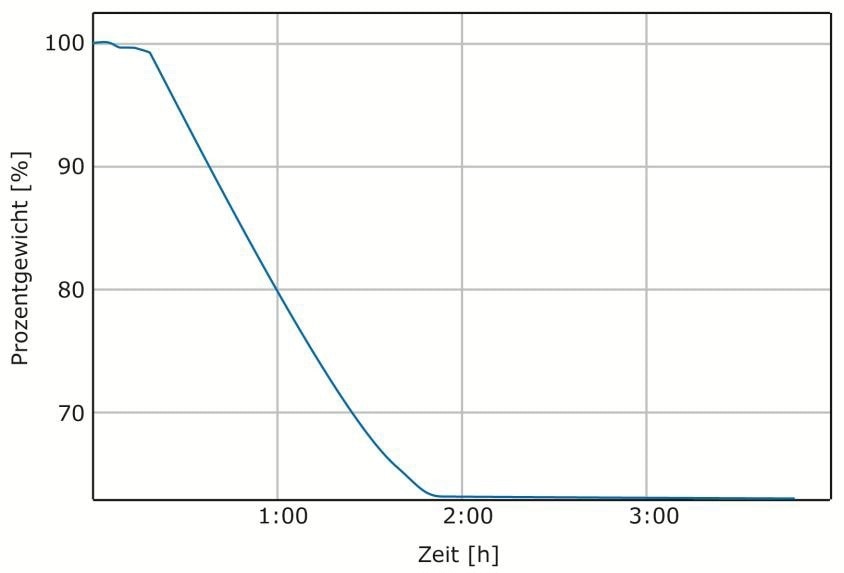
Figure 2. Typical measurement curve LOI
A benefit of the thermogravimetric method refers to the fact that the samples, after having been heated, are not submitted to ambient air; however, because of the selected weighing and measurement process it just represents an approximation to the ISO standard. Presently, it is not possible for the method to be adapted to further parameters which is partially due to the required high temperature (LOI1050) resp. to the large sample weights (LOI200 with 1 kg sample volume). Validation of the process continues to be a difficult task because of the lacking reference material.
Determination of the Carbon Content
Determination of Total Carbon
Part 2 of the ISO 21068 series also explains the analysis of silicon carbide and its carbon content. A careful differentiation has to be performed between the total carbon content and the SiC-bound carbon content. Based on the relation of the two bondings, different analysis methods have been stipulated in the standard. There are several ways of determine the overall carbon content in silicon carbide samples, which differ in the combustion method (resistance or induction furnace) and in the detection method. Coulometric, gravimetric and conductometric methods do not need calibration. It is possible for determining the carbon content by measuring the weight, the conductivity or the electric charge. The requirement of chemicals is high for these detection methods; moreover, the market does not offer adequate instruments.
Alternative procedures allowed by the standard include the use of elemental analyzers with resistance furnace (ELTRA CS-580) or induction furnace (ELTRA ELEMENTRAC CS-d) as well as detection with infrared cells. A combined usage of the detectors and a combination of the two combustion technologies are realized in ELTRA’s CS-d analyzer (Figure 3).

Figure 3. ELTRA’s ELEMENTRAC CS-d features the unique Dual Furnace Technology
Resistance furnaces with ceramic tube work at lower temperatures than induction furnaces. The ceramic combustion tubes permit a maximum temperature of 1,500 °C. To safely oxidate the overall carbon in the silicon carbide to CO2, resistance furnaces require the addition of accelerators such as tin powder or lead borate. Due to a temperature of approximately 2,500 °C, the oxidation of silicon carbide usually takes place more reliably and more rapidly in an induction furnace; furthermore, the measurement results present fewer variations (see Table 2). Whereas the accelerators for the resistance furnaces are employed for oxidation (lead borate) or local temperature rise (tin powder), the metal accelerators employed in induction furnaces are needed to guarantee combustion in the first place, since silicon carbide has no proper electric conductivity.
Table 2. Comparison of 3 measurements (Ctotal) of BAM S003 in an induction furnace and resistance furnace / % by mass
| Induction furnace CS-i |
Resistance furnace CS 580 with tin powder |
Certified value |
| 29.95 ± 0.05 |
29.82 ± 0.07 |
29.89 ± 0.07 |
A clearer definition of the calibration methods for the different procedures would be a welcome extension of the standard. Though [1-2] mention ideal calibration materials, the calibration with calcium carbonate or graphite is only described in detail for the detection method with thermal conductivity cells (chapter 5.4.5). The results presented in Table 2 were achieved through calibration with graphite.
Determination of Free Carbon
This parameter can be effortlessly determined with elemental analyzers. The standard stipulates the use of an analyzer with resistance furnace and infrared detection; however, the direct determination is restricted (chapter 6.4.1). As the upper limit for the free carbon content is provided with 2%, analysis exclusively by combustion, for example of the Euronorm standard CRM 781-1 with an informative value of 37.22% is not allowed. Thus, characterization of free carbon by direct combustion is basically limited to pure silicon carbide. To measure extremely low concentrations, the standard specifies the use of a quartz tube (as is used, for instance, in ELTRA’s CW-800 analyzer). The analyzer described under the “Determination of Total Carbon” section in this article is not ideal for this application; because of the usage of different furnaces, ceramic tubes cannot simply be exchanged for quartz tubes. Due to their permeable structure, ceramic tubes are not ideal for the determination of extremely low concentrations. Table 3 presents the results of a standard-compliant analysis of the reference material BAM-S003 and additionally of the Euronorm 781-1 standard, performed with ELTRA’s CW-800. The standard was also measured with an analyzer with ceramic combustion tube (ELTRA CS-580). The results in Table 3 obviously support the usage of a furnace with quartz tube when examining low carbon concentrations. If the concentrations are adequately high, analyzers with ceramic tubes will also be able to provide meaningful measurement results. In this case, the calibration of the elemental analyzer is of immense importance. Whereas higher concentrations can be effortlessly calibrated with pure calcium carbonate (12% carbon) or graphite (100% carbon), low concentrations can only be calibrated either with a synthetic carbon standard or an expensive reference material.
Table 3. Measurement results of free carbon concentration with different methods at 850 °C in % by mass
| Standard |
Furnace with quartz tube |
Furnace with ceramic tube |
Certified value |
| BAM S003 |
0.045 ± 0.005 |
0.01 ± 0.001 |
0.0493 |
| CRM 781-1 |
36.99 ± 0.1 |
37.1 ± 0.1 |
37.22 (informative) |
Determination of the Silicon Carbide Content
The SiC content can be established from the difference between total carbon and free carbon content; however, this is only allowed when the free carbon content is 50% or less of the total carbon content. This applies, for instance, to the BAM S-003 standard but not to CRM 781-1.
A direct analysis is possible, if the relation between bound and free carbon is more than 25%. If the CRM 781-1 sample is freed of surface carbon (Table 3), it can be directly analyzed with an induction or resistance furnace (Table 4).
Table 4. Determination of bound SiC (with induction furnace) in CRM 781-1 after oxidation at 850 °C in % by mass
| Standard |
Carbon content after combustion in induction furnace |
Calculated bound carbon content |
| CRM 781-1 (pre-heated) |
10.9 ± 0.1 |
11.03 |
Analysis of Oxygen and Nitrogen
The third part of the DIN EN 21068 series focuses on the determination of nitrogen and oxygen. In contrast to the determination of carbon species, the sample will have to be heated in an inert gas flow (helium) in order to measure its nitrogen and oxygen content. The inert gas fusion method has been established for a number of years and is also used for examining metals. It is possible to realize the stipulated temperature of approximately 2,800 °C only in an electrode/impulse furnace. A lower and an upper electrode apply voltage to the graphite crucible comprising of the sample which has earlier been purged with inert gas. The analysis quality is powerfully influenced by the power line available (approximately 5 kW recommended) and the purity of the flux used (nickel baskets or capsules). These materials are essentially employed for melting point reduction in order to discharge the gasses present in the silicon carbide from the molten sample.
Table 5 presents the results for the BAM S-003 standard obtained with ELTRA’s ONH-p analyzer. The analysis was performed with a 5 kW power line and pre-edged nickel baskets. Good compliance with the certified/informative values was achieved after calibration with a certified steel standard. Primary substances (such as KNO3) can also be employed for calibration; however, these substances will first have to be dissolved and diluted, followed by drying them in nickel capsules. The measuring principle does not permit direct analysis of liquid standards. The inert gas fusion procedure can be effortlessly applied to the analysis of other ceramics with high nitrogen (example, Si3N4) or oxygen (example, SiO2, ZrO2) content.
Table 5. Analysis of oxygen and nitrogen content in standard BAM SOO3
| O measured |
O certified |
N measured |
N (informative value) |
| 942 ± 20 ppm |
909 ± 35 ppm |
108 ± 15 ppm |
93 ± 22 ppm |
Conclusion
A comprehensive analysis of silicon carbide and mixtures containing silicon carbide in accordance with the DIN EN ISO 21068 standard series involves sophisticated technical equipment. If the determination of metals, not elaborated on in this article, is also considered, the additional use of spectrometers (ICP, OES, XRF) is called for. The market provides analyzers for standard-compliant analysis of carbon concentrations; however, varied analysis specifications need different configurations (induction furnace, combustion in ceramic or quartz glass tube). Nitrogen and oxygen are reliably and easily determined with analyzers employing inert gas fusion. For a future update of the standard, a more extensive treatment of thermogravimetric methods would be greatly recommendable. Modern TGA analyzers not only minimize human errors via automization, but also provide manifold possibilities to characterize SiC and related products, thanks to flexible use of carrier gas and temperatures.
References and Further Reading
- Römp Chemie Lexikon, 10th edition (2002)
- EN ISO 21068 (2008), Parts 1, 2, 3

This information has been sourced, reviewed and adapted from materials provided by ELTRA GmbH.
For more information on this source, please visit ELTRA GmbH.