During titrations, it is crucial to select the right titrant. If the solubility of the formed compounds is lower (their oleophilic properties is greater), the potential jump of the titration curve is steeper and larger. This effect should be leveraged when formulations are titrated. (Additives can result in a significant flattening of the potential jumps.)
In general, titrants with a concentration of 0.005 mol/L are often preferred, and a concentration of 0.02 mol/L is selected only in exceptional cases.
The equation below gives the weight of reagents required for 1 L:
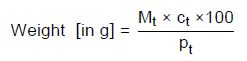
Mt—Molar mass of the titrant
ct—Concentration of the titrant; in this case, it is 0.005 or 0.02 mol/L
100—Conversion factor (L)
pt—Purity of the titrant in %
The needed amount of titrant (including slight excess) is weighed exactly and dissolved in distilled water, warming mildly if required. The substances do not include 100% active substances. Water is often the chief by-product. The water mass fraction can be up to 8% and is very hard to eliminate.
When weighing in the titrant, it is vital to consider these circumstances. Up to 1 L of the titrant should be made with distilled water at 20 °C. Determination of the titer is performed against an anionic or cationic surfactant. If the solution inside the buret and the reagent bottle is not stabilized, the titer also remains unstable (surfactants tend to adhere to surfaces and this is the case specifically for cationic surfactants). Therefore, the same reagent bottles and other equipment (for example, buret) should be used and the solution should be allowed to stand for one day before the titer is determined.
Preparation of c(TEGO®trant A100) = 0.005 mol/L
Approximately 2.12 g of TEGO®trant A100 weighed into a glass beaker, with an accuracy of 0.1 mg, is dissolved in about 150 mL of water. Distilled water is used to quantitatively transfer this solution to a 1 L volumetric flask, where it is filled up to the mark. The leaflet that comes with the TEGO®trant includes detailed information on the process.
Preparation of the Comparison Standard Solutions from Sodium Dodecyl Sulfate (SDS)
Since raw substances can contain certain impurities like water, it is suggested that the purity of the raw material is considered while calculating the weigh-in.
About 1.44 g of sodium dodecyl sulfate weighed into a glass beaker, with an accuracy of 0.1 mg, is dissolved in about 200 mL of water. Water is used to quantitatively transfer this solution to a 1 L volumetric flask, which is filled up to the mark. The contents of the flask are mixed carefully.
It is vital to note down the exact weight of the sample, as it is required for the subsequent titer calculation.
Titer Determination of the TEGO®trant A100
Into a glass beaker, 10.0 mL of the corresponding sodium dodecyl sulfate standard solution is pipetted. Then, 5 mL of methanol, 75 mL of water, and 10 mL of the buffer solution with pH = 3.0 are added. The sample solution is then stirred thoroughly and titrated with the corresponding TEGO®trant A100 solution as the titrant. The instrument settings given in the table below are used for the titration:
| . |
. |
| Pause |
30 s |
| Signal drift |
50 mV/min |
| Measuring point density |
4 |
| Min. increment |
10.0 µL |
| Stop volume |
20 mL |
| EP recognition |
all |
The results of the titration can be used only if at least one equivalence point is identified. Otherwise, additional titrations should be carried out.
Calculation of the Titer
In general, a threefold determination is always preferred. The resulting mean value is calculated to four decimal places.
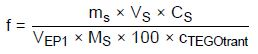
f—Titer of the titrant
VEP1—Titrant consumption in milliliters
mS—Sample weight of SDS standard in grams
VS—Added volume of SDS solution in milliliters; in this case, this value is 10.0
CS—Active substance content of the SDS used in %; in this case, this value is 99.2%
MS—Molecular weight of reference substance; in this case, this value is 288.4 g/mol
100—Conversion factor due to %
cTEGOtrant—Theoretical concentration of the titrant in mol/L; in this case, this value is 0.005 mol/L
Preparation of c(Sodium Dodecyl Sulfate) = 0.005 mol/L
In the case of anionic titrants, titer cannot be determined in the usual way as there are no appropriate primary standards. In general, cationic surfactants are quaternary ammonium compounds that cannot be made with the purity needed for a primary standard. The degree of quarternization of these compounds would have to be 100%, but this is never the case. Moreover, the majority of these compounds are extremely hygroscopic. Thus, due to water uptake, the active substance content varies each time the container is opened.
As it is not possible to determine a titer in the normal sense, the standard solutions are made by highly accurate weighing of sodium dodecyl sulfate.
SDS weighing exactly 1.44 g is added to a glass beaker and dissolved in about 250 mL of distilled water. The solution is then quantitatively rinsed into a 1 L volumetric flask using distilled water, and 10 mL of w(HCHO) = 35% is added to it. The flask is then filled up to the mark with distilled water.
Formaldehyde is added to avoid bacterial decomposition of the titrant without any adverse effect on the titration of the surfactant. The disinfecting activity of the specified quantity is enough to maintain the titer stable for a period of at least three months.
Thorough mixing is ensured by adding a magnetic stirring bar to the flask and the solution is stirred on a magnetic stirrer to ensure minimum foam formation. Then, the titrant can be transferred to the buret.
Preparation of c(DOSS) = 0.02 or 0.005 mol/L
As it is not possible to determine a titer in the normal sense, the standard solutions are made by highly accurate weighing of dioctylsodium sulfosuccinate.
DOSS weighing exactly 2.22 g for 0.005 mol/L or 8.89 g for 0.02 mol/L is added to a glass beaker and dissolved in about 250 mL of distilled water. The prepared solution is then quantitatively rinsed into a 1 L volumetric flask using distilled water, and 10 mL of w(HCHO) = 35% is added. Then, the flask is filled up to the mark with distilled water.
pH Ranges for Some Detergents
Anionic Surfactants
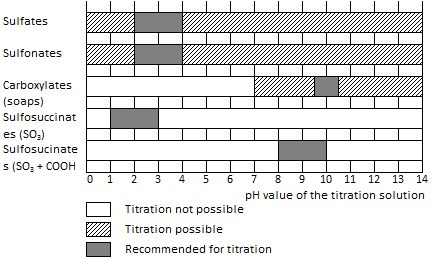
Figure 1. Titration recommendations for anionic surfactants. Image Credit: Metrohm AG
- For samples that contain both betaines and sulfosuccinates, titration of the sulfosuccinates is performed at pH = 3.0.
- To determine sulfosuccinates, the pH values given below are adjusted for titration
- sulfonate and carboxylate group at pH = 10.0
- sulfonate group at pH = 2.0
Cationic Surfactants
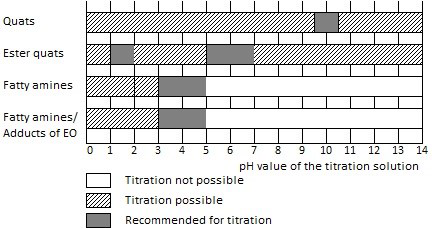
Figure 2. Titration recommendations for cationic surfactants. Image Credit: Metrohm AG
- An additional titration is performed at pH = 3.0 to determine the degree of quaternary amines. This helps obtain the sum of the tertiary starting amine and the quaternary ammonium compounds.
- The ester quats included in softeners are often titrated at pH = 2.0. But some ester quats show the greatest stability at pH = 5 or 7. Thus, they should be determined at these pH values. In this case, the pH must be adjusted soon after the sample is weighed. Even mild alkaline conditions could rapidly result in ester cleavage, making the ester to lose its surfactant properties. This would end up in error-prone results.
- It is not possible to titrate betaines and amphoteric surfactants. But they can hinder the determination of other surfactants if they exist in the protonated form (at pH = 0 to 1).
Theory Behind the Electrode
The membrane composition (ionophore/plasticizer) of the surfactant ISE electrode, which is a PVC liquid membrane electrode, is optimized exclusively to determine ionic surfactants. The electrode potential is the result of a unique interaction between the ion carrier contained in the PVC membrane and the analyte ions (surfactants) from the analysis solution.
In an equilibrium reaction, such an interaction causes a potential crossing of the analyte ions from the analysis solution into the membrane and, thus, results in a difference in electrical potential at the phase boundary analysis solution/membrane. This difference can be measured against a reference electrode at zero current, or potentiometrically.
The concentration of the analyte ions governs the extent of ion transfer from the analysis solution into the membrane. The Nernst equation, given below, describes the relationship between the concentration of the analyte ions and the resulting electrical potential:
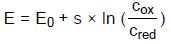
In the above equation, “s” denotes the electrode slope. It is ideally ca. 59 mV per concentration decade for monovalent anions and cations at a temperature of 25 °C. Practically, lower slopes are often observed. Due to a range of conditions (membrane composition, exclusive properties of the surfactants like boundary activity, substantivity [or the tendency to absorb on surfaces], the formation of micelles), it is not always possible to predict the Nernstian behavior for the surfactant electrodes. In practice, this implies that:
- the electrode is not ideal for the direct determinations of potentiometric concentration
- the titration should always be assessed with the inflection point of the S-shaped titration curve. End-point titrations are often not to be recommended.
Preparation, Maintenance, and Storage of the Surfactant Electrode
- The electrode is stored under dry conditions.
- It is best to condition the electrode with two to three titrations, the results of which should be ignored.
- A soft paper towel moistened with methanol is used to eliminate the adherent deposits. For sample changer operation, the electrodes are immersed in methanol for some time, while stirring continuously.
- The electrode (PVC membrane) reacts with almost all organic solvents. Hydrocarbons, tetrahydrofuran, chloroform, MIBK, acetone, etc. corrode the electrode. High proportions of ethanol (20%) or methanol (30%–40%) contained in the solvent reduce the service life of the electrode.
- Under normal conditions, the electrode can be used to perform thousands of titrations. A reduced potential range and flatter titration curves are proof of a decrease in the electrode’s reaction behavior. Such an electrode can be regenerated briefly by immersing it in a sodium dodecyl sulfate solution (0.005 mol/L) for 30 minutes. It is advisable to replace the electrode if this step is not helpful.
Analysis
The dynamic equivalence titration (DET) is the most ideal one as it enables the most rapid titrations and ensures ultimate reproducibility. An amount of sample corresponding to the consumption of at least 10 mL of titrant is weighed into a beaker, and about 50 mL of distilled water is then added.
The titration is started once 10 mL of buffer solution and 5 mL of methanol are added. To ensure accuracy, while using concentrates and raw materials, a dilution should first be made. Approximately 5%–20% vol. methanol is added to avoid micelle formation. Then, an aliquot of the initial dilution is used for the titration (consider the methanol fraction of the initial dilution).
Anionic Surfactants
- In general, a cationic surfactant at pH = 3.0 is used to titrate anionic surfactants. Refer to Figure 1 for special cases.
- The TEGO®trant A 100 must be used to titrate soaps (i.e. sodium or potassium salts of higher fatty acids) at pH values of more than 10. Other cationic titrants generate poor titration curves that cannot be assessed usually. In the case of mixtures, soaps and anionic surfactants are determined as a sum. When the pH value decreases, the fraction of the soaps determined by titration turns much smaller. Complete separation is realized through acidification of the sample to pH = 2.0 (the sample must be allowed to stand for 13 to 30 minutes to enable the reaction to run to completion). During the titration with TEGO®trant A 100, only the anionic surfactants are then determined. For the sum titration, the pH value depends on the sample used and must be identified by preliminary titrations (pH = 10 to 13). Two potential jumps are achieved, where the second one is used to determine the sum of the soaps and anionic surfactants content together.
Cationic Surfactants
- An anionic surfactant with pH = 10.0 is usually used to titrate cationic surfactants. Refer to Figure 2 for special cases.
- Samples containing amine hydrochlorides are titrated at pH = 3.0. The titration is carried out at pH = 10.0 if the content without amine hydrochlorides is needed.
Calculation
The following equation is used for known formulations or raw materials:
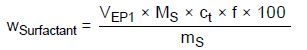
wsurfactant—Content of surfactant in %
VEP1—Amount of titrant consumed in milliliters to reach the first EP
MS—Molar mass of surfactant in g/mol
ct—Concentration of the titrant in mol/L
f—Titer of the titrant
100—Conversion factor due to %
mS—Sample weight in milligrams
If the molar mass of the surfactant under analysis is not known or if the total surfactant content must be determined (without using an average molar mass), the result of the analysis can also be specified in mmol surfactant per 100 g sample or as sulfur equivalent, as shown in the equations below:
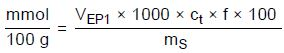
mmol/100 g—Content in mmol surfactant per 100 g sample
VEP1—Amount of titrant consumed in milliliters to reach the first EP
1000—Conversion factor mol to mmol
ct—Concentration of the titrant in mol/L
f—Titer of the titrant
100—Conversion factor due to 100 g
mS—Sample weight in milligrams
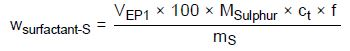
wSurfactant-S—Content of surfactant as sulfur equivalent in %
VEP1—Amount of titrant consumed in milliliters to reach the first EP
MSulfur—Molecular weight of sulfur, 32.064 g/mol
100—Conversion factor due to %
ct—Concentration of the titrant in mol/L
f—Titer of the titrant
mS—Sample weight in milligrams
Calculation for cationic surfactants in formulations (3.2):
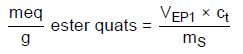
meq/g ester quats—Molar equivalence ester quats per 1 g of sample
VEP1—Amount of titrant consumed in milliliters to reach the first EP
mS—Sample weight in grams
ct—Concentration of the titrant; in this case, it is 0.02 or 0.005 mol/L
Calculation for cetylpyridinium chloride (3.2.6):
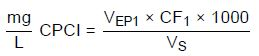
VEP1—Amount of c(SDS) = 0.005 mol/L consumed in milliliters to reach the first equivalence point
VS—Sample volume in milliliters
CF1—1.79005 (conversion factor: 1 mL c(SDS) = 0.005 mol/L ≙ 1. 79005 mg CPCl)
1000—Conversion factor due to grams to milligrams
Equivalence Calculation
1 mL anionic surfactant 0.005 mol/L
= 1.9985 mg Tego®trant
= 1.7901 mg HDPCl
= 2.2405 mg Hyamine® 1622
1 mL cationic surfactant 0.005 mol/L
= 1.4419 mg sodium dodecyl sulfate
= 2.2229 mg DOSS
Sources and Further Reading
- R. Schulz, R. Gerhards, Optimization of the potentiometric titration of ionic detergents, American Laboratory 26/11, (1994) 40-44 and International Laboratory 24/10, (1994) 10-14.
- Schulz, R. Gerhards, H.-D. Käseborn, Die potentiometrische Bestimmung von Anionentensiden in Rinse-off-Produkten, SÖFW-Journal 120/13, (1994) 776-783.
- R. Schulz, R. Gerhards, A new titrant for the potentiometric titration of anionic detergents, Tenside/Detergents, issue 1, 1995.
- S. Selig, The potentiometric titration of surfactants and soaps using ion-selective electrodes, Fresenius J. Anal. Chem. 300, (1980) 183-188.
- G. C. Dilley, Determination of anion active matter in detergents by potentiometric titration, Analyst 105, (1980) 713-719.
- K. Kosswig, H. Stache, Die Tenside, Carl Hanser Verlag, Müchen/Wien, 1993, ISBN 3-446- 16201-1.
- Stache, K. Kosswig, Tensid-Taschenbuch, 3. Auflage, Carl Hanser Verlag, München/Wien, 1990, ISBN 3-446- 15704-2.
- M. Schmitt, Analysis of Surfactants, Surfactant Science Series Vol. 40, Marcel Dekker Inc., New York, 1992, ISBN 0-8247- 8580-0.
- D. C. Cullum, Introduction to Surfactant Analysis, Blackie Academic & Professional, London, 1994, ISBN 0-7514-0025-4.
- ASTM D 4251-89, Standard Test Method for Active Matter in Anionic Surfactants by Potentiometric Titration.
- ASTM D 5070-90, Standard Test Method for Synthetic Quaternary Ammonium Salt in Fabric Softeners by Potentiometric Titration.
- R. Schulz, Th. Goldschmidt AG, Zentralbereich Forschung/Analytik, Goldschmidtstrasse 100, D-45127 Essen, Fax +49 201 173 19 97, Numerous personal communications regarding his work and lecture material.
- R. Schulz, P. Bruttel Bestimmung ionischer Tenside in Mundpflegeprodukten SÖFW-Journal 124/3, (1998) 138-146.
- R. Schulz, P. Bruttel Analytik ionischer Tenside in Haarpflegeprodukten SÖFW-Journal 125/2, (1999).

This information has been sourced, reviewed and adapted from materials provided by Metrohm AG.
For more information on this source, please visit Metrohm AG.