Tetracyclines (TCs) are among the most commonly employed antibiotics in the animal production sector, mainly utilized to prevent infection, treat diseases and protect animal growth.
There is a risk that the overuse of antibiotics such as tetracyclines might lead to residue violations in foodstuffs derived from animals, for example, milk and meat. These residues can pose a risk to human health and wellbeing.
Tetracycline residues in food may result in toxicity, as well as a number of side effects, including allergic reactions, and may also cause bacterial resistance, which can then be transferred to humans.
Regulatory bodies globally have established maximum residue levels (MRLs), which are tolerances of veterinary drugs in foods, to protect human health. The European Union, China and Canada have set what they refer to as “maximum residue levels of drug residues in foods.” The US, meanwhile, calls these “tolerances.”1,2
Employing techniques that are sensitive and highly selective enough to ensure accurate monitoring of tetracyclines based on regulatory requirements is a crucial tool in safeguarding human health. This article demonstrates a UHPLC/MS/MS method for the rapid, sensitive and quantitative analysis of tetracyclines in meat.
To boost lab productivity, the QS-Works high throughput autosampler was utilized. The instrument’s temperature-controlled trays, large capacity and robotic arms all help facilitate unattended operation for extended time periods.
The QS-Works also lets labs run up to 600 samples in a sequence, improving the efficiency of testing labs.
The method developed here demonstrated the sensitivity, recovery, precision linearity and selectivity necessary for analyzing tetracyclines in meat at low tolerance levels set by different regulatory bodies around the globe.
Experimental
Hardware and Software
Chromatographic separation was carried out on a PerkinElmer QSight® LX50 UHPLC system. Detection was attained utilizing a PerkinElmer QSight® 420 MS/MS detector.
A high-capacity QS-Works autosampler was employed to augment lab productivity. The Simplicity 3Q™ software platform was utilized to facilitate the instrument control, data acquisition and subsequent data processing.
LC Method and MS Source Conditions
Table 1 displays the LC method and MS source parameters. Table 2 shows various reaction monitoring mode (MRM) transitions of the tetracyclines. A minimum of two MRM transitions was monitored for each analyte. This was done to reduce the number of false positive and negative results in the method.
The optimization of MS/MS parameters, including factoring variables such as choice of parent ions and product ions(CCL2), entrance voltages (EV) and collision energies (CE), was accomplished via the infusion of standards.
Optimization of source conditions was achieved via t-infusion of neat standards with LC flow. The acquisition MS method was performed on the basis of those optimized conditions.
Table 1. LC Method and MS Source Parameters. Source: PerkinElmer Food Safety and Quality
| |
LC and MS Condition |
| Mobile phase A |
Water (contains 0.1% Oxalic acid) |
| Mobile phase B |
Acetonitrile (contains 0.1% formic acid) |
| Column |
PerkinElmer Quasar SPP Pesticides, 100 × 4.6 mm; 2.7 μm, (N9306880) mm |
| Column oven temperature |
20 °C |
| Autosampler temperature |
20 °C |
| Injection volume |
5 μL |
| ESI voltage (positive) |
5100 V |
| Drying gas |
120 |
| Nebulizer gas |
250 |
| Source temperature |
350 °C |
| HSID temperature |
250 °C |
Table 2. MRM Transitions. Source: PerkinElmer Food Safety and Quality
| Compound name |
Precursor ion/Da |
Product ion/Da |
CE/V |
EV/V |
CCL2/V |
| Oxytetracyline |
461.3 |
426.3
201.2 |
-21
-49 |
31
25 |
-136
-180 |
| Tetracycline |
445.3 |
410.3
427.3 |
-22
-15 |
29
27 |
-144
-124 |
| Chlortetracycline |
479.2 |
154.0
444.2 |
-34
-25 |
26
29 |
-136
-164 |
| Doxytetracycline |
445.3 |
154.1
154.0 |
-35
-34 |
29
26 |
-140
-136 |
Standards and Sample Preparation
Every reagent, solvent and diluent utilized were LC/MS grade. All TC standards - which were stored at -20 °C in freezer conditions to prevent their degradation - were obtained from Sigma-Aldrich® Inc.
The stock and mixed drug solutions for the spiking and calibration stages were prepared in methanol for every TC. To prevent degradation of the standards, all the working standards and stock were stored in freezer conditions until they were ready to be used.
To make a homogenized sample, the following procedure was followed:
- A representative meat sample was cut into small segments and then blended.
- A 1 g aliquot of the homogenized meat sample, along with an 8 mL measure of 0.1 M aqueous EDTA solution were placed in a 50 mL centrifuge tube and vortexed.
- A 17 mL measure of acetonitrile was then placed in the same tube and vortexed.
- This tube was subsequently placed in freezer conditions and maintained at -20 °C for 30 minutes.
- This was followed by centrifuging for 10 minutes at 4000 rpm.
- An 8 mL measure of supernatant was then transferred into a 50 mL clean centrifuge tube, with 5 mL of hexane added for sample defatting.
- The tube was vortexed for 3 minutes.
- Following this, the solution was then centrifuged for 1 min at 4000 rpm.
- The 3 mL of lower phase was then transferred into a new 15 mL centrifuge tube.
- This was evaporated to dryness by means of blowing nitrogen over it at a temperature of 50 °C.
- The dried extract was reconstituted by dissolving it in a 1 mL of methanol/water (30/70, v/v) solution, which contained 0.01 M oxalic acid.
- Prior to injection onto the LC/MS/MS system, these sample extracts were vortexed and filtered by means of 0.2 µm PTFE syringe filters.3
Results and Discussion
This study’s objective was to evaluate the performance of a high-capacity QS-Works autosampler with a validated sample preparation method to determine the levels of tetracyclines in a meat sample.
Employing a high throughput autosampler, such as QS-Works, enhances testing lab productivity and efficiencies.
Where food testing labs have a substantial number of samples to analyze, QS-Works allows for the rapid processing of samples while simultaneously enhancing data quality and consistency due to the use of a robotic sampling unit (Figure 1).

Figure 1. QS-Works autosampler. Image Credit: PerkinElmer Food Safety and Quality
Either the maximum residue levels (MRLs) set out for tetracyclines in foods by Health Canada or the tolerances established by the US FDA were utilized as the reference target levels, shown as X in Table 3. The detection threshold - the ‘yes/no’ screening level - should be at or below 0.5X.
The chromatograms (XIC) of 4 tetracyclines with good peak resolution can be seen in Figure 2. Identification and confirmation were carried out by monitoring two ion transitions.
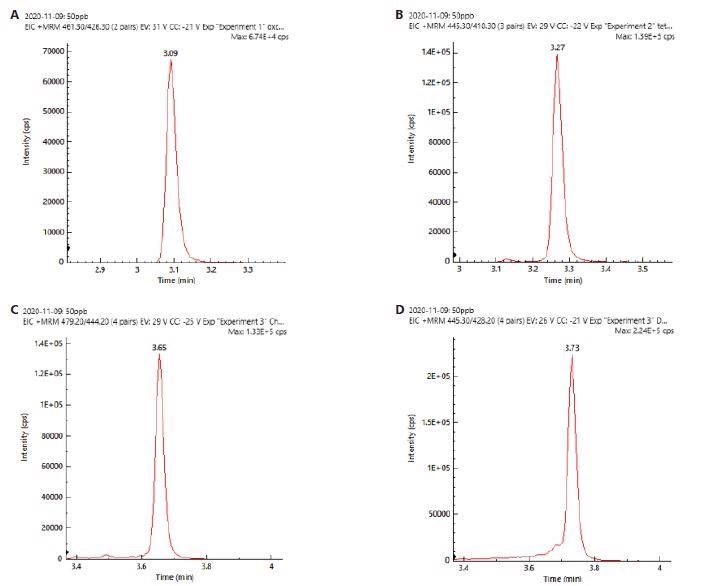
Figure 2. XIC chromatograms of a) oxytetracycline, b) tetracycline, c) chlortetracycline, d) doxytetracycline. Image Credit: PerkinElmer Food Safety and Quality
This allowed for higher selectivity and specificity in identification. The ion ratios were computed by calculating peak area for less intense ion to peak area for more intense ion to generate ion ratios.
Due to the diversity and complexity of food sample matrices, sample matrix effects (MEs) are the main concerns for LC/MS/MS method development.
ESI is known to be susceptible to ionization suppression of analytes where charge-competing matrix components are present. It has been established that matrix-induced enhancement effects occur in ESI, and this can also introduce a large bias in quantification.
To overcome sample MEs, several approaches have been used. These included sample dilution, use of stable isotope internal standards, matrix-matched (MM) calibration, standard addition, sample clean-up and use of high efficiency columns for improved separation.
By comparing the slopes of calibration curves obtained from the meat sample matrix against the slopes obtained from reagent-only (RO) samples, the sample matrix effects (MEs) were evaluated.
Sample ME (%) for each analyte was calculated by the percentage difference between the slopes. MEs for most of the compounds were less than 20% (Table 3).
To overcome matrix effects and achieve a reduction in variation in analytical results, matrix-matched calibrations were employed in this study for the quantification of every analyte.
The calibration was carried out utilizing both matrix-matched (MM) and reagents-only (RO) standards. Every calibration curve built from both the RO and meat sample matrices displayed good linearity with correlation coefficient (R²) larger than 0.99. Examples of typical calibration curves can be seen in Figure 3 and Figure 4.
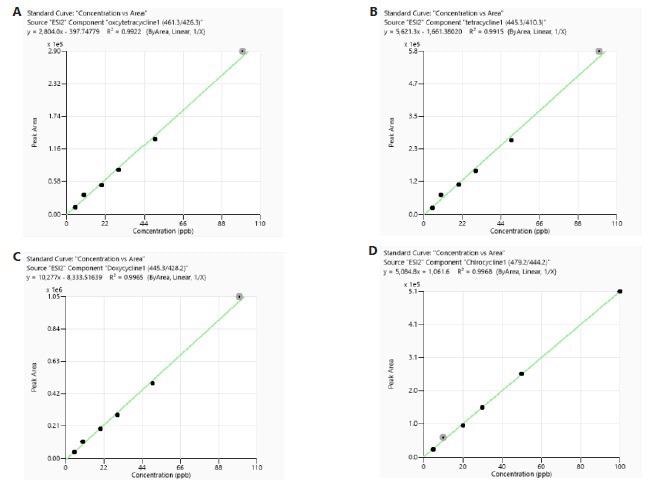
Figure 3. Calibration curves of tetracyclines obtained from standards prepared in reagent only. Image Credit: PerkinElmer Food Safety and Quality
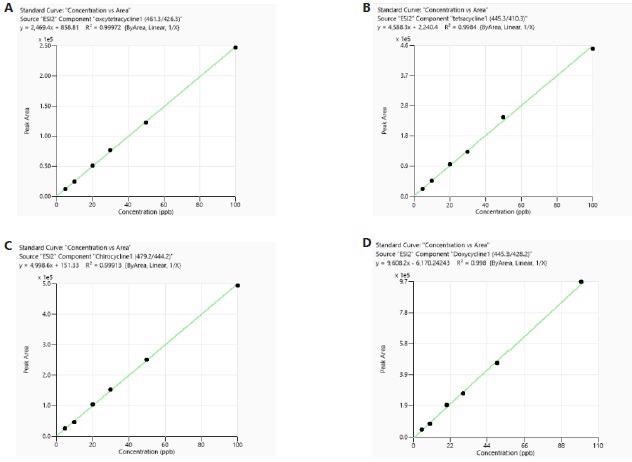
Figure 4. Calibration curves of tetracyclines obtained from standards prepared in meat sample matrix. Image Credit: PerkinElmer Food Safety and Quality
Carryover was assessed by means of injecting the reagent blank after a high concentration standard. No carry-over was observed in any of the experiments that were performed.
To avoid chelation of tetracyclines by metal ions of LC parts or column body and maintain good peak shape and reproducibility over the run, oxalic acid was added into aqueous mobile phase.3
The method displayed good precision with RSD of less than 5% for five replicates. The effects of using oxalic acid in mobile phase and reconstitute solvent on the reproducibility are shown in Table 3.
The reproducibility and peak shape of every analyte was improved by the addition of oxalic acid into mobile phase and reconstitute solvent. The LOQ for each tetracycline analyzed was found to be lower than their permitted tolerance level in meat samples.
This shows that this application displays more than adequate sensitivity for tetracycline analysis in meat at tolerance level.
The estimated limits of quantification (LOQs) for the TCs were based on signal/noise (S/N) ratio of 10. The LOQ for all the drugs studied are 1.5 ± 1.0 µg/L.
The findings showed that the developed method is suitable for use in the fast screening and quantification of tetracyclines in meat samples.
The absolute recoveries of tetracyclines were in the range of 70-120% (Table 3). Utilizing a deuterated tetracycline can be used to compensate for losses of tetracyclines with this extraction process in the future.
Table 3. Results of retention time, matrix effect (ME), linearity, recovery, and reproducibility (%RSD). Source: PerkinElmer Food Safety and Quality
| Compound name |
Level
(ng/g)
Tolerance
(x) |
RT (min) |
ME
(%) |
Linearity
(R2) |
Recovery
(%) |
RSD with oxalic acid
(%) (n=5) |
RSD without oxalic acid
(%) (n=5) |
| Oxytetracyline |
200 |
3.09 |
86 |
0.9984 |
70.0 |
4.6 |
11.0 |
| Tetracycline |
200 |
3.26 |
80 |
0.9997 |
116.0 |
4.9 |
16.0 |
| Chlortetracycline |
200 |
3.65 |
96 |
0.9991 |
87.3 |
1.9 |
9.5 |
| Doxytetracycline |
200 |
3.73 |
94 |
0.9980 |
71.6 |
1.5 |
8.0 |
Conclusion
The development of a UHPLC/MS/MS method utilizing a PerkinElmer high throughput QS-Works autosampler and LX 50 UHPLC coupled to a QSight 420 MS/MS system displayed good sensitivity for the identification and quantification of tetracyclines in a homogenized meat sample.
The low levels of detection achieved by this method can facilitate the support of low regulatory limits in routine screening and quantitation analysis applications.
References
- List of Maximum Residue Limits (MRLs) for Veterinary Drugs in Foods, Health Canada, August, 2017. https://www.canada.ca/en/health-canada/services/drugs-health-products/veterinary-drugs/maximum-residue-limits-mrls.html. Accessed November, 2020.
- Standard Method Performance Requirements (SMPRs®) for Screening and Identification Method for Regulated Veterinary Drug Residues in Food. SMPR2018_010.pdf (aoac.org). Accessed November, 2020.
- Aurélien Desmarchelier, Sébastien Anizan, Mai Minh Tien, Marie- Claude Savoy & Cindy Bion (2018) Determination of five tetracyclines and their epimers by LC-MS/ MS based on a liquid-liquid extraction with low temperature partitioning, Food Additives & Contaminants: Part A, 35:4, 687-695, DOI: 10.1080/19440049.2018.1427894
Acknowledgments
Produced from materials originally authored by Saba Hariri, Abir Khaled, Avinash Dalmia and Feng Qin from PerkinElmer, Inc.

This information has been sourced, reviewed and adapted from materials provided by PerkinElmer Food Safety and Quality.
For more information on this source, please visit PerkinElmer Food Safety and Quality.