The extraction of lithium from lithium-ion batteries (LIB) is becoming increasingly crucial due to the growing adoption of electrification, notably in the transportation industry.
Being labeled a critical raw material by the European Union (EU),1 escalating raw material costs, heightened demand, and legally mandated recycling quotas are prompting extensive research endeavors and the establishment of recycling facilities.
These initiatives, centered on reclaiming lithium alongside other valuable battery components, primarily concentrate on lithium recovery. A possible target product that could be used as a raw material in battery production is lithium carbonate.
The recovery of lithium in current recycling processes is still very challenging and is either not carried out at all or only with low recovery rates.2
Addressing this challenge, the Institute for Metallurgical Process Technology (IME) at RWTH Aachen University is exploring a method for early lithium recovery from the battery’s active material.3,4
To achieve this goal, the battery material intended for recycling—either entire cells or batteries already shredded—is subjected to a specific thermal pretreatment.
This process serves a dual purpose: firstly, it eliminates organic substances from the battery cells’ electrolytes, binders, and separators. Secondly, it facilitates phase changes in the lithium present within the battery by adjusting targeted process parameters. The desired phase for lithium is lithium carbonate, which forms through ongoing reduction reactions.
Following this, a wash-out process employing deionized water is employed for the selective retrieval of lithium from the active material. During this phase, the lithium carbonate that is produced dissolves.
However, due to the thermal pretreatment, lithium fluoride is also produced, and depending on the process parameters chosen, some of this salt is brought into the solution. The lithium salt product is then recovered from the solution, for example, via the evaporation of the water.
In evaluating the process parameters of thermal pretreatment and leaching, as well as assessing the produced product, it is crucial to consider the purity and the specific type of lithium compound present.
Since the target phase in the case described is lithium carbonate, the TIC (total inorganic carbon) measurement with the multi N/C analyzer provides a reliable and fast way to compare the carbonate contents within a series of tests and to draw conclusions about the ratio of lithium fluoride to lithium carbonate.
Additionally, the measurement of the TOC value (total organic carbon) allows the user to check for unwanted contamination of the sample with organic residues from the thermal treatment process.
In this article, Analytik Jena’s multi N/C 2100S was utilized to gauge carbonate concentrations. The measurements involved aqueous samples derived from a series of tests conducted at IME.3
These samples were obtained from the water-washing process of four active compounds subjected to different thermal pretreatments. Further verification was achieved through fluorine measurements, while XRD images of the precipitated salts were captured.
These images allow for qualitative conclusions to be made regarding the phase distributions of lithium fluoride and lithium carbonate.
Materials and Methods
The TIC determination was conducted using the multi N/C 2100S and the autosampler AS 60, following the TIC determination method outlined in DIN EN 1484.
Samples and Reagents
- Four samples of active mass post-water washing, each from a distinct thermal pretreatment process (610 °C, 1-hour holding time, under different atmospheres: N2, N2 + 2.5% O2, N2 + 5% O2, and CO2 atmospheres)
- 10 % phosphoric acid used for automated TIC determination
- Calibration standard solutions for TIC comprised of sodium carbonate and sodium hydrogen carbonate dissolved in water
Sample Preparation and Measurement
The liquid samples underwent a 1:8 dilution with deionized water to reduce potential effects from metal cations present in the matrix. These samples were then placed in vials and positioned on the sample rack.
For direct TIC measurement, a representative 500 µL sample aliquot was injected into the analyzer’s TIC reactor using a microliter syringe.
Subsequently, a 10% phosphoric acid aliquot was automatically introduced into the TIC reactor. This acid converts CO32- (carbonate ion) into HCO3- (bicarbonate, the dissolved form of CO2). The dissolved CO2 is released from the solution through purging with a clean carrier gas (such as pure oxygen or synthetic air devoid of hydrocarbons and CO2).
After appropriate drying and purification steps, the measurement gas was directed to the detector. Quantification was achieved using non-dispersive infrared spectrometry, employing a focus radiation NDIR detector.
Calibration
The multi-N/C analyzer underwent calibration using standard solutions of sodium carbonate and sodium hydrogen carbonate in a 50:50 mix to cover concentrations ranging from 0.25 to 25 mg/L TIC.
All calibration solutions adhered to DIN EN1484 standards and were prepared accordingly. A linear calibration function was applied for this calibration process.
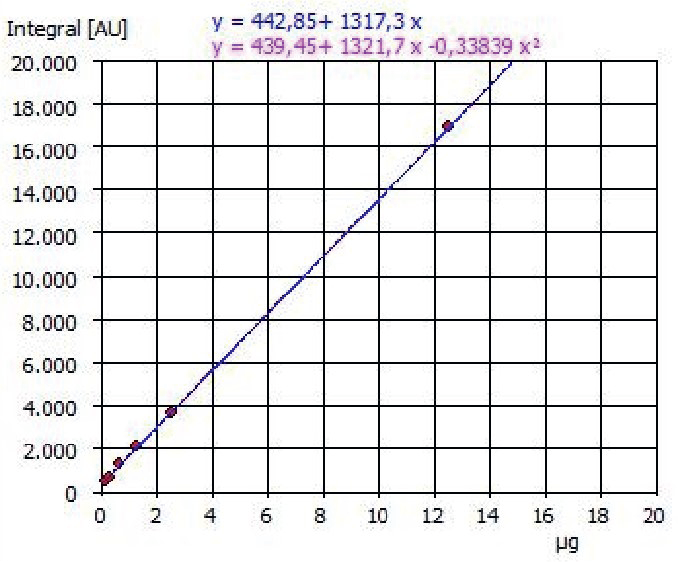
Figure 1. Example of a calibration curve TIC 0.25 - 25 mg/L. Image Credit: Analytik Jena US
Table 1. Method parameters for multi N/C 2100S. Source: Analytik Jena US
| Parameter |
multi N/C 2100S |
| Method of determination |
TIC |
| Sample digestion |
10 % H3PO4 |
| Number of replicates |
min. 2, max. 3 |
| Autosampler, rack, and vial size |
AS 60, 60 position rack, 8 mL sample vials |
| Rinse cycles with sample |
3 |
| Injection volume |
500 µL |
| Integration time |
240 s |
Results and Discussion
In Table 2 and Figure 2, examples of analytical results are provided based on multiple TIC measurements conducted from a sample vessel.
Additional analyses were conducted for qualitative comparison. Fluorine analyses using an ion-selective electrode were carried out. Moreover, semi-quantitative assessments of lithium carbonate and lithium fluoride fractions from the precipitated lithium salt were determined via XRD measurements.
However, determining carbonate content via XRD measurements had its drawbacks.
Firstly, it is significantly more time-consuming due to an additional step required for lithium salt precipitation. Additionally, the entire process of conducting an XRD measurement, including sample preparation, takes considerably longer compared to TIC determination from a solution. Furthermore, phase fraction determination via XRD is semi-quantitative.
In contrast, TIC measurements from a solution offer distinct advantages. This method stands out for its precision, low standard deviation, and rapid measurement times.
Table 2. Comparison of the results of TIC and fluorine determination based on undiluted original sample and semiquantitative XRD evaluation of the precipitation product. Source: Analytik Jena US
| Sample ID |
Atmosphere for
thermal pretreatment |
Results TIC
± SD [mg/L] |
F determination
[mg/L] |
Li2CO3 in precipitated
salt [%-wt]
(XRD) |
LiF in precipitated
salt [%-wt]
(XRD) |
| 1 |
N2 |
47.5±0.46 |
36.6 |
81.5 |
18.5 |
| 2 |
CO2 |
60.2±0.54 |
27.9 |
86.4 |
13.6 |
| 3 |
N2 + 2.5 % O2 |
38.4±0.01 |
34.8 |
73.3 |
26.7 |
| 4 |
N2 + 5 % O2 |
29.5±0.26 |
30.0 |
79.2 |
20.8 |
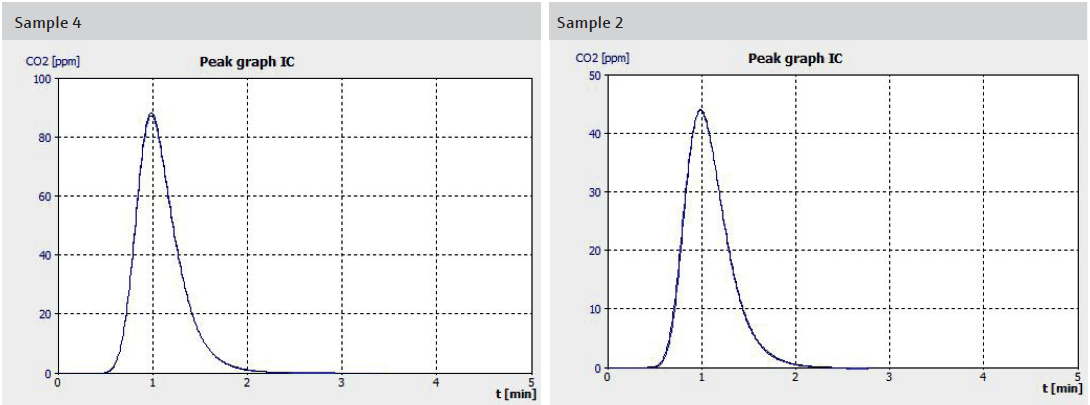
Figure 2. Examples of measurement curves of samples with different content of carbonates. Image Credit: Analytik Jena US
The measurement curves depicted in Figure 2 showcase the remarkable consistency of the measured values across multiple injections from a sample vessel. This high reproducibility enables the quantitative determination of lithium recovery rates in the aqueous eluate solution through TIC analysis.
When comparing this with the semi-quantitative XRD method used to determine lithium fluoride and lithium carbonate proportions in the resulting precipitate, a clear relationship emerges: Samples with higher lithium carbonate content in the salt product exhibit elevated TIC readings in solution, while those with lower carbonate content show lower readings.
For the comparison of the samples with each other and for the estimation of the process success regarding the yield of lithium carbonate during thermal pretreatment and the corresponding lithium recovery in the elution step, the TIC measurement thus offers a suitable and time-saving alternative in comparison to the semi-quantitative phase determination by means of XRD.
Conclusion
The TIC determination performed using the multi-N/C series serves as an effective method for comparing lithium carbonate generation within battery recycling. The measurement data demonstrates its reliability in qualitatively assessing lithium carbonate recovery.
Simultaneously, this method significantly saves time compared to the semi-quantitative phase analysis conducted via XRD on the salt precipitation product. Utilizing the multi N/C 2100S TOC analyzer with direct injection technique alongside the AS 60 autosampler presents a notable advantage in terms of minimal sample consumption per analysis.
Typically, less than 5 mL of sample is enough for a triplicate determination of the TIC parameter. An added benefit lies in the capability to analyze the sample for organic residues (TOC) dissolved in the solution. The detection of such residues is crucial as they could adversely impact the quality of the lithium salt product.

Figure 3. multi N/C 2100S. Image Credit: Analytik Jena US
Table 3. Overview of devices, accessories and consumables. Source: Analytik Jena US
| Article |
Article number |
Description |
| multi N/C 2100S |
450-200.100-2 |
TOC analyzer with direct injection technique |
| AS 60 |
450-126.682 |
Autosampler for multi N/C 2100S |
| Alternative: |
|
|
| multi N/C 3100 |
450-200.500-2 |
TOC analyzer with flow injection technique |
| AS vario |
450-900.140 |
Autosampler for multi N/C 3100 |
| Rack 72 positions |
450-900.141 |
Accessory for AS vario |
References
- Blengini GA, El Latunussa C, Eynard U, et al.: Study on the EU‘s list of critical raw materials (2020): Final report. Publications Office of the European Union, Luxembourg, https://doi.org/10.2873/11619
- Harper GDJ, Kendrick E, Anderson PA, et al. (2023) Roadmap for a sustainable circular economy in lithium-ion and future battery technologies. J Phys Energy 5:21501. https://doi.org/10.1088/2515-7655/acaa57
- C. Stallmeister, B. Friedrich: Influence of Flow-Gas Composition on Reaction Products of Thermally Treated NMC Battery Black Mass, Metals 2023, 13(5), 923 https://doi.org/10.3390/met13050923
- Stallmeister C, Schwich L, Friedrich B (2020) Early-Stage Li-Removal - Vermeidung von Lithiumverlusten im Zuge der Thermischen und Chemischen Recyclingrouten von Batterien. In: Holm O, Thomé-Kozmiensky E, Goldmann D et al. (eds) Recycling und Rohstoffe. Thomé-Kozmiensky Verlag GmbH, Neuruppin, pp 544–557

This information has been sourced, reviewed and adapted from materials provided by Analytik Jena US.
For more information on this source, please visit Analytik Jena US.