Organic aerosols, which are formed by the oxidation of volatile organic molecules, account for up to 90% of global atmospheric aerosol mass.

Image Credit: TOFWERK
Oxygenated VOCs with low and/or extremely low vapor pressures are particularly significant to the climate. They are essential in generating atmospheric aerosols by condensing onto pre-existing aerosol particles or generating new particles.
The synthesis and proliferation of particles begins with producing highly oxygenated organic molecules (HOMs) via autoxidation. In addition to organic components, inorganic species contribute significantly to the production and growth of atmospheric particles.
Sulfur oxides (SOx) are major air pollutants that directly impact the Earth's atmosphere. The most common of these is sulfur dioxide (SO2), which is emitted mostly by human activities (e.g., fuel combustion) and natural emissions (e.g., volcanic eruptions).
Once in the atmosphere, SO2 is rapidly oxidized, producing sulfur trioxide (SO3). This molecule is a key intermediate in the production of sulfuric acid, which is known to play an important role in atmospheric new particle generation.
SO3 can also react with organic surfaces, forming sulfur-containing compounds that significantly impact the physicochemical properties of aerosols.
As the atmospheric lifetime of gaseous SO3 is very brief due to its rapid reactivity with water dimer molecules (to generate sulfuric acid), its reaction with other atmospherically significant chemicals is expected to be negligible.
Laboratory and field studies, however, point to the creation and presence of gaseous sulfur-containing compounds in the atmosphere.
Following these findings, a few studies have looked at the gas-phase synthesis mechanism of sulfur-containing compounds from SO3 interactions with organics, particularly carboxylic acid found in the environment.
Analytical Challenges in Analysis of Sulfur-Containing Compounds in the Atmosphere
Understanding the evolution of sulfur-containing compounds in the atmosphere is crucial for controlling the physicochemical mechanisms that cause particle production. Due to their extremely low vapor pressure and acidity, sulfur-containing compounds are expected to play an essential role in the processes that produce new particles.
Accurate characterization of such species presents an analytical problem for researchers due to the relatively comparable weights of compounds containing oxygen and sulfur, making them virtually indistinguishable in standard mass spectrometry analysis.
In many cases, precise spectral fitting techniques are required to resolve overlapping peaks at the same integer mass, although these mathematical approaches may fail to determine the true composition of isobaric species.
Addressing Analytical Challenges with Vocus HR
The Vocus High Resolution (HR) online chemical ionization mass spectrometer, which employs multi-reflection technology to produce a 5.5-meter flight path in a compact frame, can distinguish such chemicals in real time for laboratory or field investigations.
This study simulated air conditions to assess whether the newly developed Vocus HR could distinguish sulfur-containing compounds from other closed-shell products, providing new insight into these crucial species.
Experimental Setup
To test the Vocus HR system, gaseous species were created in an 18-liter Pyrex glass aerosol flow tube reactor (12 cm i.d. × 158 cm length) from the O3/OH-initiated oxidation of α-pinene in the presence of SO2 at room temperature and atmospheric pressure.
Ozone was produced at a stable concentration of 80 ppb by passing 0.6 standard liters per minute (SLPM) of synthetic air through a UV lamp (Ozone Generator Model 610, Jelight Company, Inc, Irvine, USA), which was measured using a Thermo 49C analyzer.
The flow reactor was constantly injected with 5 ppb of α-pinene and SO2 in stepwise concentrations of 0.5-10 ppb. The carrier gas was an 80:20 mixture of nitrogen and oxygen (total flow 18 SLPM), with a reaction time of approximately 60 seconds.
The Vocus HR was connected to an Aerodyne atmospheric pressure chemical ionization (CI) intake using nitrate (NO3-) ion chemistry.
NO3- ions were generated from a 65% nitric acid solution (Sigma-Aldrich) that was continuously flushed with 2 standard cubic centimeters per minute (sccm) of pure N2 and then mixed with 25 LPM of N2 serving as a sheath flow (for a total flow of 34 LPM), which was further ionized with a soft X-ray photoionizer.
Enhanced Detection of Oxygenated Compounds Using Vocus HR
To assess the Vocus HR's detection capabilities, oxidation products from α-pinene oxidation with and without SO2 were tested using a steady-state flow tube configuration.
This reaction mechanism was chosen since it is widely characterized and produces diverse oxidation products. It produces molecules with a wide range of molecular weights, from mildly oxidized monomers to highly oxidized dimers.
Figure 1 shows that the Vocus HR has a higher mass resolving power (20,000 - 25,000 Th Th−1) than traditional online mass spectrometers (10,000 Th Th−1), allowing for precise identification of isobaric substances.
Doubling the mass-resolving power improves the robustness of peak fitting, allowing for more precise and consistent identification and quantification of the compounds of interest.
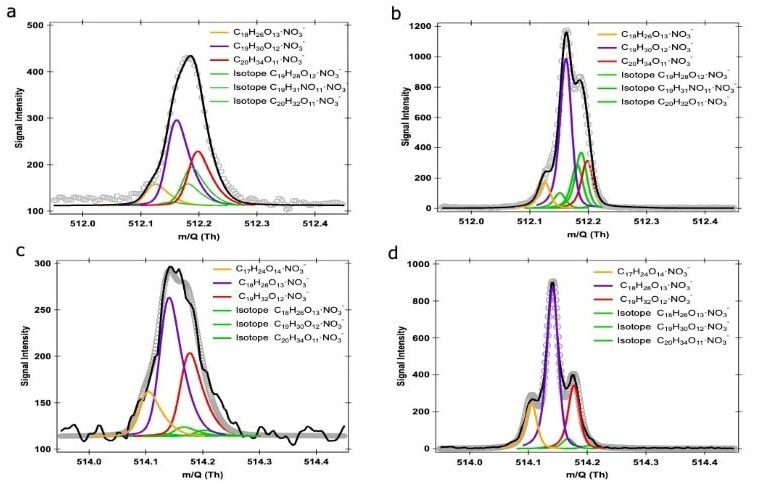
Figure 1. An example of traditional HR peak fitting. A potential peak fitting at m∕Q 512 and 514 Th of products generated during the OH/O3 initiated oxidation of α-pinene, utilizing the nitrate-based CI-Vocus HR at a resolving power of (a, c) 10,000 Th Th−1 and (b, d) 22,000 Th Th−1. Image Credit: TOFWERK
Detection of Sulfur-Containing Compounds Using the Vocus HR
The Vocus HR solely allows the detection of C10H16O5S species grouped with NO3–, as seen in Figure 2.
When using a standard time-of-flight spectrometer, additional information is required to reliably detect the existence of such chemicals and identify them, particularly at low concentrations.
Although identification is possible, the spectrum overlap between C10H16O5S and C10H16O7 is so significant that obtaining quantitative information will be challenging due to ambiguous peak fitting, resulting in poor signal separation.
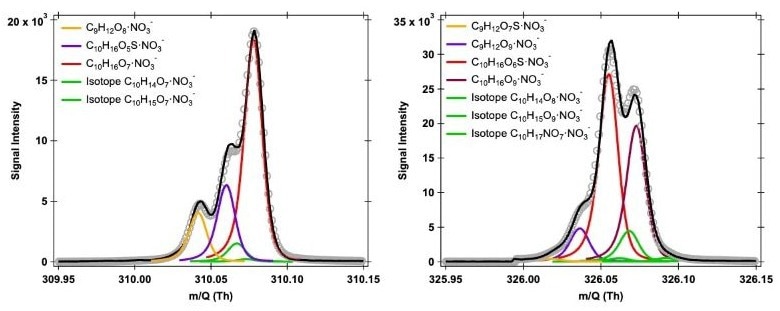
Figure 2. Potential peak fitting at m∕Q 310 and 326 Th of oxygenated products generated during the OH/O3 initiated oxidation of α-pinene in the presence of SO2, utilizing the nitrate-based CI-Vocus HR at a resolving power of 22,000 Th Th−1. Image Credit: TOFWERK
The Vocus HR detects 75 sulfur-containing species out of about 250 during the OH/O3-initiated oxidation of α-pinene in the presence of 5 ppb SO2.
Figure 3 shows the subsequent synthesis of gaseous sulfur-containing species, implying that sulfur trioxide may affect the oxidation of biogenic compounds, resulting in the formation of extremely low vapor-pressure molecules that can contribute to the formation of new particles in the atmosphere.
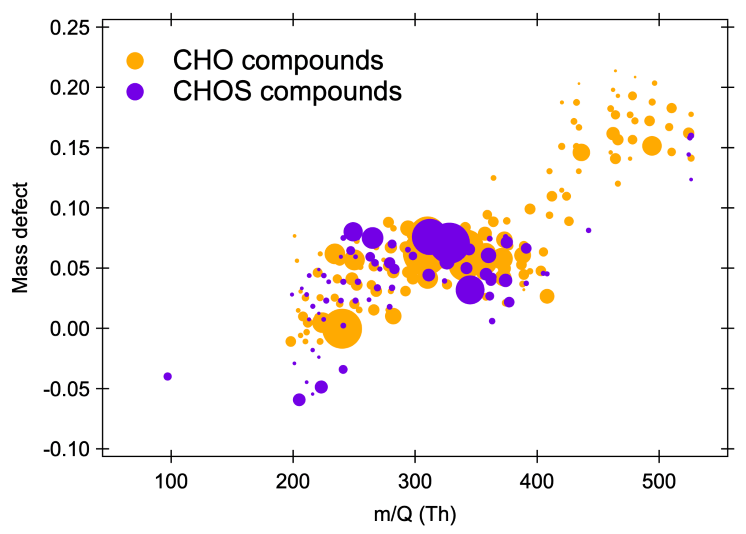
Figure 3. Mass defect plot of non-sulfur-containing (orange) and sulfur-containing (purple) species measured by the nitrate-based CI-Vocus HR generated via the OH/O3-initiated oxidation of α-pinene in the presence of 5 ppb of SO2. The x-axis represents the mass-to-charge ratio of the neutral analyte; the y-axis represents the corresponding mass defect, which is the difference between their exact mass and nominal mass; and the size of the circle represents the square root of the signal intensity measured for each ion. Image Credit: TOFWERK
Conclusion
The combination of sensitivity and selectivity provided by CI reactors, as well as the Vocus HR's enhanced mass resolving power, enables researchers to separate sulfur and oxygen-containing compounds in real time, allowing them to better understand these crucial precursors of particle formation in the atmosphere.
Analysis of these species could lead to improved regulatory frameworks, reducing a leading source of pollution-related death in humans and negative environmental effects.
Acknowledgments
Produced from materials originally authored by Matthieu Riva, Katie Schmidt, Vasyl Yatsyna, and Felipe Lopez-Hilfiker from TOFWERK, Switzerland.

This information has been sourced, reviewed and adapted from materials provided by TOFWERK.
For more information on this source, please visit TOFWERK.