Sponsored by ESSLABReviewed by Olivia FrostAug 28 2025
Regular oil changes are not just routine maintenance. They are essential for keeping an engine running smoothly and efficiently. Engine oil acts as the lifeblood of the vehicle, lubricating moving parts and cooling components, cleaning residues, and preventing internal corrosion.

Image Credit: ESSLAB
Engine oil degrades over time when exposed to heat, oxygen, friction, and pollutants. This degradation diminishes the oil's ability to protect the engine and, if not corrected, can cause engine damage.
The Total Acid Number (TAN) is a key measure of oil degradation, indicating how acidic the oil has become as it ages. As oil degrades, it reacts with heat and oxygen in the engine, producing acidic chemicals that raise the oil's acidity level.
Rising acidity can damage components, shortening engine life and reducing performance. Understanding how oil degrades and the impact of TAN is key to understanding why regular oil changes are necessary to keep engines running well.
Why Regular Oil Changes are Important
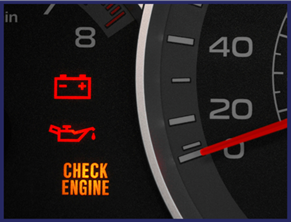
Image Credit: ESSLAB
As the oil ages, its ability to lubricate the engine and reduce friction declines, which can create several problems, including:
- Increased Wear and Tear: When oil thickens or loses its lubricating ability, friction between components increases, accelerating wear and tear. Over time, this can cause crucial engine components like pistons, cylinders, and bearings to wear down, resulting in expensive repairs and decreased engine performance.
- Corrosion: As oil degrades, the acidity increases. High TAN levels indicate that oil is becoming more acidic, which can cause corrosion of engine parts. This is particularly damaging to metal components, which may rust, pit, and lose structural integrity.
- Sludge Formation: Degraded oil can form sludge that blocks vital oil channels. When this occurs, oil cannot flow efficiently, resulting in overheating, poor lubrication, and an increased risk of engine failure.
- Loss of Cooling Efficiency: Engine oil also plays a crucial role in absorbing heat to prevent overheating. However, degraded oil thickens and loses its ability to flow correctly, reducing cooling capacity and putting additional load on the engine.
The Role of TAN in Oil Degradation
TAN is determined by titrating an oil sample with a base, usually potassium hydroxide (KOH) or sodium hydroxide (NaOH). The amount of base required to neutralize the acids in the oil sample is directly proportional to its acidity level. The higher the TAN value, the more acidic and damaged the oil is.
The Analytical Titration Process for Measuring TAN
Measuring TAN is critical for identifying the state of engine oil and whether it is time for an oil change. The titration procedure for measuring TAN entails adding a base to the oil sample to neutralize the acids, and it provides a precise way to assess the oil's acidity.

Image Credit: ESSLAB
- Sample Preparation: A precise sample of old engine oil is obtained for testing. Typically, the oil sample is weighed and dissolved in a solvent to facilitate titration. A combination of alcohol and toluene is frequently used as a solvent to separate acidic chemicals and improve titration accuracy.
- Titration with Base: The sample is titrated with a standardized base solution, such as KOH or NaOH. The base neutralizes the acids in the oil sample, increasing the pH of the solution.
- Monitoring the Endpoint: A pH indicator, such as phenolphthalein, changes color when the solution's pH swings from acidic to neutral. The endpoint is reached when the solution is neutral or slightly basic, indicating that all acidic chemicals in the sample have been neutralized.
- Calculation of TAN: To calculate the TAN, measure the volume of base needed to neutralize the acids in the oil sample, the concentration of the base, and the sample's weight. The formula used is:
TAN=V×N×56.1W\text{TAN} = \frac{V \times N \times 56.1}{W}TAN=WV×N×56.1
Where:
- V = Volume of titrant used (in mL)
- N = Normality of the titrant (concentration in equivalents per litre)
- W = Weight of the oil sample (in grams)
- 56.1 = A factor relating moles of KOH to acid groups in the sample.
The Role of Reference Materials in TAN Measurement
To ensure the precision and reliability of TAN measurements, reference materials are used during the titration procedure. These items are required to calibrate the titrant solution and ensure the correctness of the test findings.
Reference materials are substances with known qualities, such as a specified acid value, that can be used to ensure titration accuracy. In TAN testing, reference materials serve the following purposes:

Image Credit: ESSLAB
Titrant Standardization: The titrant solution is standardized using reference materials before evaluating the oil sample. A reference material with a known acid value is titrated with the base, confirming that the titrant is at the proper concentration.
Calibration for Equipment: Burettes and pH meters are examples of reference materials used to calibrate titration equipment. This guarantees that the instrument provides precise measurements throughout the titration procedure.
Accuracy Verification: Following the titration of the oil sample, a reference material with a known TAN is titrated in parallel to ensure accurate results. If the oil sample results match the known value of the reference material, it means the method was carried out appropriately.
Quality Control: Reference materials maintain uniformity between different batches of oil samples and over time. By employing reference materials with recognized values, laboratories can ensure consistent and reproducible results, allowing vehicle owners to make educated decisions regarding their oil change plan.
Industry Insight: The Growing Importance of Oil Analysis
In recent years, the automotive industry has increasingly emphasized oil analysis as part of preventive maintenance programs.
Manufacturers increasingly rely on routine oil testing to determine the best timing for oil changes. Oil analysis not only helps extend the engine's life but also increases fuel efficiency and lowers emissions by ensuring the engine is constantly running on clean, effective oil.
Oil technology has advanced, allowing oil to stay longer and withstand higher temperatures than ever before.
Even advanced oils deteriorate over time, so regular testing of indicators such as TAN is essential to know when a change is due. By monitoring oil quality, automobile owners can make decisions to avoid costly engine repairs and extend the life of their vehicles.
Conclusion
Using reference materials to calibrate the titration process ensures the precision and reliability of TAN measurements, hence preserving engine efficiency and lowering emissions.
In today's competitive automotive business, oil analysis is vital for preventing damage, boosting fuel efficiency, and extending vehicle life. Regular oil changes and analysis are investments in the long-term health and reliability of your engine.
ARO Scientific - Certified Reference Materials
ASTM 664-24 - Standard Test Method for Acid Number of Petroleum Products by Potentiometric Titration: defines a potentiometric titration with a pH electrode as the endpoint. This approach is unaffected by the sample's color. It is compatible with petroleum products, lubricants, biodiesel, and biodiesel blends.

Image Credit: ESSLAB
ASTMD974 - Standard Test Method for Acid and Base Number by Color-Indicator Titration: defines a photometric titration that uses an optical sensor to indicate the result. This test method is ideal for light-colored petroleum products and lubricants.

This information has been sourced, reviewed and adapted from materials provided by ESSLAB.
For more information on this source, please visit ESSLAB.