Precisely quantifying metal oxide samples’ chemical compositions with X-ray photoelectron spectroscopy (XPS) may be adversely impacted by adventitious carbon contamination, which is often found on the surface of the sample.
This article outlines the way in which a Nexsa™ XPS system from Thermo Scientific™, coupled with a dual-mode monatomic and gas cluster ion source, was utilized to examine the effects of using ionized argon clusters to clean metal oxides.
XPS (also known as ESCA) is an analytical method that is often employed to determine chemical information from the top-most 1–10 nm of a sample. This may include a small surface layer of carbon contamination, which can impact on the quantification of the sample.
The majority of XPS systems are supplied with an ion source that may be used to remove surface material for the depth profiling or cleaning of samples. This ion source generally produces monatomic ions of a noble gas, usually argon.
When monatomic argon ion bombardment is used to clean metal oxides, any contamination is removed from the surface. However, these ions can also penetrate the underlying sample, resulting in chemical damage even at low ion energies.

Thermo Scientific MAGCIS dual-mode ion source.
This damage is usually observed as reduction of the oxide and is caused by the removal of oxygen and preferential sputtering. Thermo Scientific’s™ MAGCIS™ argon cluster ion source can address this difficulty, however.
It enables the sputtering of the surface via large cluster ions. Because these do not penetrate the oxide surface, this enables chemical state information to be maintained while still removing any contamination from the sample.
The dual-mode MAGCIS source is also capable of generating a monatomic beam, which is ideal for profiling into a harder inorganic substrate. This ability to provide both monatomic ions and cluster ions means that the MAGCIS source is both versatile and convenient when working with a wide range of sample types.
This article explores how the cluster mode of MAGCIS can be employed to clean an oxide sample, without inducing any reduction in the remaining surface.
Experiment
A spectra from a sample of tantalum pentoxide was acquired using the Nexsa XPS instrument. Ta2O5 foil was mounted on a standard sample holder, and spectra were taken at three different areas, following the application of different cleaning methods.
Spectra were taken in the first area, in its base state with no cleaning performed. Meanwhile, in the second position, the sample was sputtered with 200 eV monatomic ions prior to analysis. Finally, in the third position, the sample was analyzed after it had been sputtered with singly ionized argon clusters.
The MAGCIS ion source is able to create argon clusters with a variety of cluster sizes and beam energies. Within this experiment, the cluster beam utilized beam energy of 4 keV, with a cluster size of 1000 atoms.
Additionally, survey spectra – that is, wide scans which cover the whole elemental range - were obtained from the areas which had no cleaning performed on them, as well as the area which was sputtered with the cluster ion beam.
Results
Figure 1 shows a comparison of the XPS survey spectrum taken from the Ta2O5 sample in its base state and cluster-cleaned state. While the two spectra are relatively alike, the base spectrum displays a considerable amount of carbon contamination on the surface, confirmed by the sizeable peak at 285 eV.
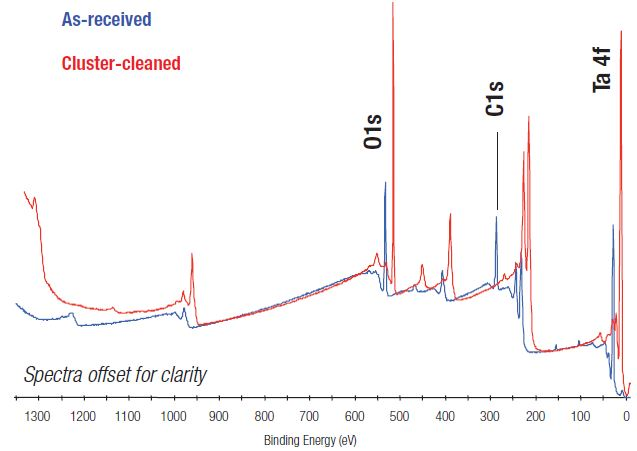
Figure 1. Comparison of XPS survey spectra before and after argon cluster ion cleaning.
Following cleaning via argon cluster beam, contamination on the surface of the sample is significantly reduced. Quantifying the spectrum indicates that the surface is Ta2O5.
Table 1. Atomic concentrations from before and after cluster-cleaning.
| Name |
‘As-received’
Atomic % |
After Cluster-cleaning
Atomic % |
| C |
50.7 |
0 |
| O |
33.7 |
71.8 |
| Ta |
11.8 |
28.2 |
| Si |
3.8 |
0 |
Table 1 highlights the atomic concentrations for the two spectra displayed in Figure 1. It is evident from both Figure 1 and Table 1 that after cluster-cleaning, the surface contamination is no longer present.
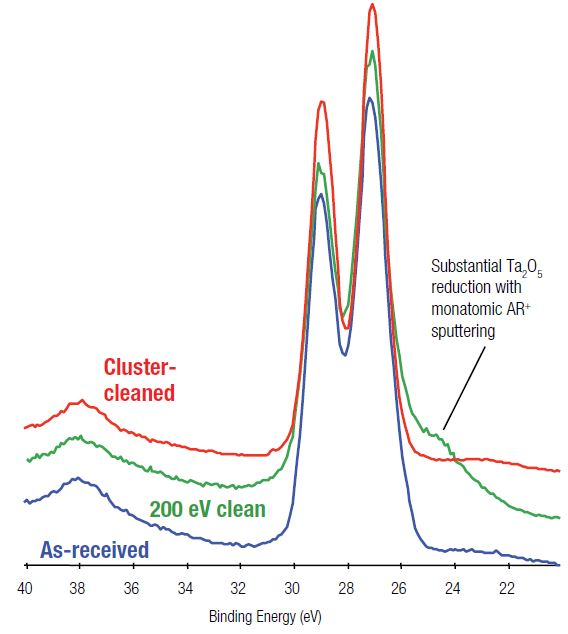
Figure 2. Comparison of Ta 4f spectra for monatomic Ar+ and argon cluster ion sputter-cleaning of Ta2O5.
Figure 2 displays a comparison of Ta 4f spectra from the three surface areas: base, following a 200 eV monatomic ion clean, and following cluster ion cleaning. The monatomic ion beam-cleaned surface displays obvious signs of reduction, confirmed by the shoulder on the low binding energy side of the doublet when compared against the base spectrum.
This corresponds to around 30% of the surface is reduced as a result of ion-induced chemical changes. A 200 eV monatomic ion beam delivers low beam energy but still brings about reduction. The use of more common monatomic ion beam energies, like 1000 eV, would result in even more damage.
Table 2. Relative intensities of Ta 4f oxide and reduced components before and after sputter-cleaning.
| Cleaning Method |
Ta 4f Oxide |
Ta 4f Reduced |
| None |
100 |
– |
| Cluster ions |
100 |
– |
| 200 eV monatomic |
70.4 |
29.6 |
It should be noted that the cluster cleaned surface does not show this reduction, confirming that the stoichiometry of the surface has been preserved after the removal of the carbon. Table 2 shows these surface compositions.
Summary
Within this experiment, a Ta2O5 sample was cleaned using both cluster and monatomic ions. Differences between XPS spectra taken after the cleaning had taken place in comparison to those taken of the non-cleaned surface are obvious.
The sample exhibited considerable surface damage (thus reducing the signal) when the MAGCIS ion source was used in monatomic mode. However, when the MAGCIS source was employed in cluster mode, no visible damage was present. The surface was still cleaned of adventitious carbon, however.
Even when low energy monatomic Ar+ ion sputter-cleaning was used, there was a considerable amount of Ta2O5 reduction, confirming that cluster ions may be vital in the analysis of metal oxide.
Gas cluster ion beams have been shown as being well suited for the successful cleaning of inorganic samples with no visible signs of oxide reduction and with no induction of chemical changes to the surface being measured.
Acknowledgments
Produced from materials originally authored by Christopher Deeks and Paul Mack from Thermo Fisher Scientific.

This information has been sourced, reviewed and adapted from materials provided by Thermo Fisher Scientific – X-Ray Photoelectron Spectroscopy (XPS).
For more information on this source, please visit Thermo Fisher Scientific – X-Ray Photoelectron Spectroscopy (XPS).