Surface Enhanced Raman Spectroscopy was used to investigate the effectiveness of two types of packaging for preserving ground pork meat from lipid oxidation.

Thermo Scientific DXR3 Raman Microscope. Image Credit: Thermo Fisher Scientific – Materials & Structural Analysis
Introduction
Lipid oxidation is a significant issue for the meat industry. This is because lipid oxidation can have a detrimental impact on not only sensory quality, in terms of odor and flavor, but it can also lead to the formation of toxic compounds like malondialdehyde (MDA) and cholesterol oxidation products, in addition to the accumulation of carbonyls, alcohols, and volatile acids.
The degree of oxidation depends on various factors such as the nature and origin of the meat, the fat content of the meat, the temperature at which the meat is stored, the availability of oxygen, and the type of packaging.1,2
Synthetic antioxidants are commonly added to meat products to prevent oxidation, but today consumers demand more natural products.3
To evaluate the antioxidant activity of many essential oils or extracts from plants or spices, such as cinnamon, rosemary, or oregano, several studies have been carried out. A new trend that has been identified is the method of developing innovative packaging by adding these natural antioxidants directly to the packaging materials.4,5,6
These materials, often films, are termed “active packaging” because they allow for the controlled release of active agents (either on the surface or in the internal atmosphere of the product) or operate by trapping the molecules responsible for detrimental effects on food quality.
However, it is essential to accurately determine the antioxidant performance of these films in order to enable the selection of the right packaging for each foodstuff.
Raman spectroscopy is a promising candidate and frontrunner, among all the techniques available, when it comes to detecting most molecular species with high sensitivity and specificity, particularly in the presence of Surface-Enhanced Raman Scattering (SERS).
SERS is the name given to a physical phenomenon that occurs in the presence of a rough nanostructured metallic surface. Thanks to the combination of a chemical effect and an electromagnetic effect, SERS causes the intensification of Raman signals from molecules adsorbed to the surface.7
Generally gold or silver are the metals used, and these can be prepared by metallic coating, electrochemical roughening, or deposition of nanoparticles (often in a colloidal form).
Though commercial solutions are available, many researchers prefer to create their own SERS substrates. The scope of this work is to assess the potential of a home-made SERS substrate, with which to compare the lipid oxidation over time in ground pork preserved in one of two packaging types: either in a low-density polyethylene (LDPE) conventional packaging or in an active packaging which contains oregano extract.
Material and Method
Fresh ground pork meat was purchased from a local supermarket in bulk, and 22 g samples of meat were placed inside 5-cm diameter polystyrene Petri dishes. Next, each sample was covered with an active film and packaged under a normal atmosphere in an LDPE bag.
Conventional LDPE films were also used to prepare reference samples. Each sample was stored in a refrigerator at 5 °C and after 0, 7, 9, 11, 14 and 16 days their lipid extracts were analyzed using a home-made SERS substrate based on silver nanoparticles deposited on a glass Petri dish.
The analyses were performed at low temperature using a special home-made cooling system, and SERS spectra were measured at -2 °C with a Thermo Scientific™ DXR3 Raman Microscope (Waltham, MA, USA) with a 532 nm laser source, a 900 lines/mm grating covering the 50-3500 cm-1 spectral range and an x10 objective (NA 0.40).
The laser power was set at 5 mW, and to acquire all data, 20 exposures of 30 each were selected. Thermo Scientific™ OMNIC™ v. 9.2 software was used for data collection and analysis. Each measurement was performed in triplicate.
The area ratio between 1655 cm-1 and 1439 cm-1 bands was calculated to follow the lipid oxidation, given that the decrease in the peak at 1655 cm-1 (stretching of C=C bonds from unsaturation) can be attributed to lipid peroxidation and the 1439 cm-1 (scissoring deformation of CH2) is not affected by it.
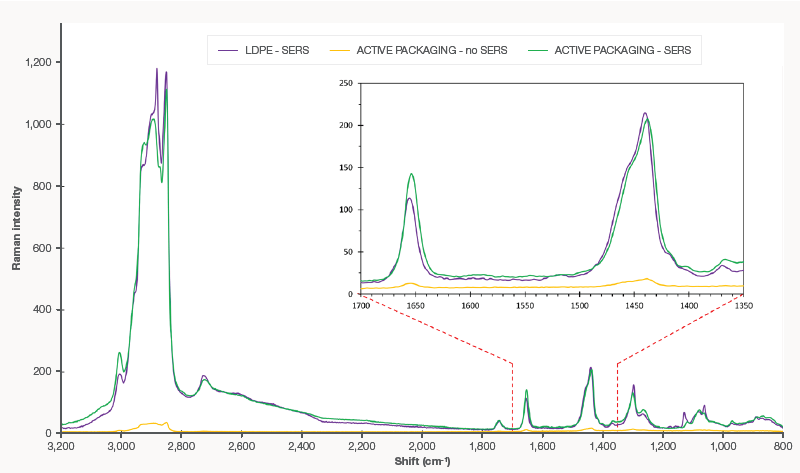
Figure 1. Conventional Raman (yellow) and SERS spectra of fat extracted from meat on the 7th day. The higher peak at 1655 cm-1 of active packaging (green) indicates effective protection in comparison with LDPE (purple). Image Credit: Thermo Fisher Scientific – Materials & Structural Analysis
Introduction
Results and Discussion
The Raman and the SERS spectra of fat extracted with n-hexane/diethyl ether (1:1) from meat are shown in Figure 1 after seven days of storage in both conventional LDPE and active packaging. The SERS spectrum shows bands of much higher intensity than the conventional Raman spectrum, where the conventional and active packaging spectra were almost identical.
It was found during the study that the difference between oxidized and fresh samples was more pronounced in the SERS spectra measured at low temperatures (data not shown). The calculation of enhancement factor (EF) for a given shift is as follows:8

Where ISERS is the signal intensity at the selected shift with the silver substrate, the following is true: IRaman is the signal intensity at the same shift without the silver substrate, the number of molecules responsible for the enhancement is NSERS, and the number of molecules in the detection volume is NRaman. The calculated enhancement factors for the two bands of interest in this study, 1439 cm-1 and 1655 cm-1, are shown in Table 1.
Table 1. EF calculated for the 1439 cm-1 and 1655 cm-1 bands. Source: Thermo Fisher Scientific – Materials & Structural Analysis
| . |
. |
| EF1439 |
1.64·107 |
| EF1655 |
8.58·106 |
The very high efficiency of the homemade SERS substrate is demonstrated, as these are on the order of a factor of 107. Higher sensitivity and, therefore, faster acquisition is signaled by using such a substrate.
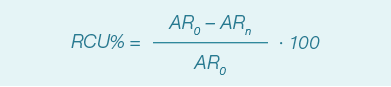
Measuring the relative change of unsaturation (RCU%) assessed the packaging efficiency, where AR0 and ARn represent the area ratio between the 1655 cm-1 and the 1439 cm-1 SERS bands respectively at day 0 and after n days of storage. The higher the level of lipid oxidation, the higher the RCU% value.
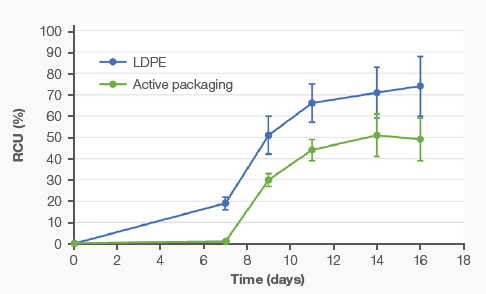
Figure 2. Relative change of unsaturation (RCU%) over time for conventional LDPE packaged meat and active packaged meat. Image Credit: Thermo Fisher Scientific – Materials & Structural Analysis
The graphical results of the RCU% obtained for the meat stored in both conventional and active packaging are shown by Figure 2. An increase in lipid oxidation over time is indicated by the values calculated in this study, which appears to accelerate from day seven of storage.
Consistently, the RCU% values are lower for the active packaging than for the conventional LDPE packaging. This result confirms that, due to their oxidation over time, this type of packaging is more effective in preventing the unsaturation of fats in ground pork.
Conclusion
With regards to monitoring lipid oxidation in packaged ground pork meat over time, the effectiveness of a home-made SERS substrate coupled with a homemade cooling system was demonstrated. The advantages of this technique, as evidenced by this study, are its speed and the minimal sample handling requirements.
An observation was made of an improvement factor of the order of 107 over conventional Raman. Finally, the greater efficacy of active packaging containing oregano extracts against lipid oxidation in ground pork was also confirmed by this study, compared to conventional LDPE packaging.
Acknowledgments
Produced from materials originally authored by Eloïse Ribette Lancelot, of Thermo Scientific France; Jesús Salafranca, of the Instituto de Investigación en Ingeniería de Aragón (I3A), and Magdalena Wrona, of the Instituto de Investigación en Ingeniería de Aragón (I3A).
References and Further Reading
1 P. A. Morrissey, D. J. Buckley, P. J. A. Sheehy, and F. J. Monahan, “Vitamin E and meat quality,” Proceedings of the Nutrition Society, vol. 53, no. 2, pp. 289–295, 1994, doi: 10.1079/pns19940034.
2 M. S. Brewer, W. G. Ikins, and C. A. Z. Harbers, “TBA Values, Sensory Characteristics, and Volatiles in Ground Pork During Long-term Frozen Storage: Effects of Packaging,” J Food Sci, vol. 57, no. 3, pp. 558–564, 1992.
3 Z. Formanek, J. P. Kerry, F. M. Higgins, D. J. Buckley, P. A. Morrissey, and J. Farkas, “Addition of synthetic and natural antioxidants to α-tocopheryl acetate supplemented beef patties: Effects of antioxidants and packaging on lipid oxidation,” Meat Sci, vol. 58, no. 4, pp. 337–341, 2001, doi: 10.1016/S0309-1740(00)00149-2.
4 C. Nerín et al., “Stabilization of beef meat by a new active packaging containing natural antioxidants,” J Agric Food Chem, vol. 54, no. 20, pp. 7840–7846, 2006, doi: 10.1021/jf060775c.
5 J. Camo, J. A. Beltrán, and P. Roncalés, “Extension of the display life of lamb with an antioxidant active packaging,” Meat Sci, vol. 80, no. 4, pp. 1086–1091, 2008, doi: 10.1016/j.meatsci.2008.04.031.
6 C. Nerín, L. Tovar, and J. Salafranca, “Behaviour of a new antioxidant active film versus oxidizable model compounds,” J Food Eng, vol. 84, no. 2, pp. 313–320, 2008, doi: 10.1016/j.jfoodeng.2007.05.027
7 B. Sharma, R. R. Frontiera, A. I. Henry, E. Ringe, and R. P. Van Duyne, “SERS: Materials, applications, and the future,” Materials Today, vol. 15, no. 1–2, pp. 16–25, 2012, doi: 10.1016/S1369-7021(12)70017-2
8 E. C. Le Ru, E. Blackie, M. Meyer, and P. Etchegoin, “Surface enhanced Raman scattering enhancement factors: A comprehensive study,” J Phys Chem C, vol 111, no. 37, pp. 13794–13803, 2007, doi: 10.1021/jp0687908.
9 M. Wrona, J. Lours, J. Salafranca, C. Joly, and C. Nerín, “Innovative Surface Enhanced Raman Spectroscopy Method as a Fast Tool to Assess the Oxidation of Lipids in Ground Pork,” Applied Sciences (Switzerland), vol. 13, no. 9, 2023, doi: 10.3390/app13095533

This information has been sourced, reviewed and adapted from materials provided by Thermo Fisher Scientific – Materials & Structural Analysis.
For more information on this source, please visit Thermo Fisher Scientific – Materials & Structural Analysis.