Feb 10 2015
A research team led by René Janssen at Technische Universiteit Eindhoven (TU/e) have succeeded in explaining the reason behind the two or three fold improvement in the efficiency of plastic solar cells when a solvent is added at the time of manufacture. This discovery will pave the way for focused plastic solar cell development.
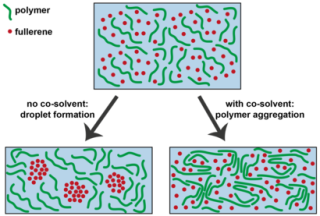 The infographic shows the effect of the co-solvent on the solution of two plastic components (polymer and fullerene molecules) during the production process of the plastic solar cell. Without the co-solvent droplets of fullerene molecules are formed which restrict the efficiency of the solar cell. With the co-solvent the polymer molecules fold much more quickly, so that droplets can't be formed. (Source: TU Eindhoven / Hans van Franeker).
The infographic shows the effect of the co-solvent on the solution of two plastic components (polymer and fullerene molecules) during the production process of the plastic solar cell. Without the co-solvent droplets of fullerene molecules are formed which restrict the efficiency of the solar cell. With the co-solvent the polymer molecules fold much more quickly, so that droplets can't be formed. (Source: TU Eindhoven / Hans van Franeker).
Generally, plastic solar cells are manufactured using polymers rather than silicon in order to convert solar energy into electricity. The use of polymers as fundamental material helps to bring down the weight as well as the cost of these solar cells, thereby making them flexible.
However, it was noticed that their efficiency only reached about 10% in comparison to the commercial silicon solar cells, which could realize around 15 to 20% efficiency.
A plastic solar cell’s effectiveness can be increased greatly with the use of additional solvents (co-solvents), which are added during production. The team has published their findings in a publication in Nature Communications.
“These co-solvents are now used in all plastic solar cells”, says TU/e professor René Janssen. “But nobody knew exactly why they have such a favorable effect on the efficiency.”
It had been established that there was a link with the solar cell morphology, which is the precise structure of two mixed plastic parts in the cell, between which electrons travel due to solar energy.
These plastic components are organic materials, which are dissolved during manufacture, following which they tend to evaporate and solidify. The strange co-solvent is added to the solvent prior to evaporation.
The TU/e researchers integrated certain optical technologies to reach a conclusive justification. Their experiments indicated that when a co-solvent was not added, large droplets were created while the plastic mixture hardened. These droplets tend to affect the movement of electrons in an adverse manner causing the efficiency of the solar cell to drop as well.
“The more co-solvent you add to the solution, the smaller the bubbles turn out to be, until they disappear completely when a specific content is reached”, says Janssen.
The TU/e researchers were able to find a solution for this issue. “There are two effects that arise during the hardening process”, says Janssen. “On the one hand the solution evaporates, and as well as that polymers take on a ‘folded’ structure. We saw that the co-solvent makes this ‘folding’ process start at a much earlier stage, which means the bubbles are ultimately no longer formed at all.” Therefore, the co-solvent behaves similar to baking powder, and enhances the structure of the mixture; however the agent on its own will not suffice.
The researchers are hopeful that their discovery will aid in the advancement of plastic solar cells’ effectiveness. “Up to now it was mainly a question of trial-and-error”, says Janssen. “But now we can predict much more accurately what is likely to work, and what isn’t.”