Jun 23 2015
For the first time, researchers at the Department of Energy's SLAC National Accelerator Laboratory have tracked the ultrafast structural changes in quadrillionths-of-a-second steps during the opening reaction of ring-shaped gas molecules. They collated the series of steps involved in the basic ring-opening reaction to form computerized animations, producing in a "molecular movie" that explains the structural changes.
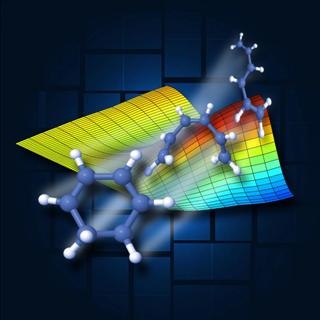 This illustration shows shape changes that occur in quadrillionths-of-a-second intervals in a ring-shaped molecule that was broken open by light. The molecular motion was measured using SLAC's Linac Coherent Light Source X-ray laser. The colored chart shows a theoretical model of molecular changes that syncs well with the actual results. The squares in the background represent panels in an LCLS X-ray detector. Credit: SLAC National Accelerator Laboratory
This illustration shows shape changes that occur in quadrillionths-of-a-second intervals in a ring-shaped molecule that was broken open by light. The molecular motion was measured using SLAC's Linac Coherent Light Source X-ray laser. The colored chart shows a theoretical model of molecular changes that syncs well with the actual results. The squares in the background represent panels in an LCLS X-ray detector. Credit: SLAC National Accelerator Laboratory
The study, carried out at SLAC's Linac Coherent Light Source, a DOE Office of Science User Facility, marks a breakthrough for a number of real-time X-ray studies of gas-based chemical reactions that play a major role in biological processes. It also demonstrates the way gas molecules are usually converted on the femtosecond scale during chemical reactions. The findings of the study were published in the June 22 edition of Physical Review Letters.
"This fulfills a promise of LCLS: Before your eyes, a chemical reaction is occurring that has never been seen before in this way," said Mike Minitti, a SLAC scientist who led the experiment in collaboration with Peter Weber at Brown University.
"LCLS is a game-changer in giving us the ability to probe this and other reactions in record-fast timescales," Minitti said, "down to the motion of individual atoms." This method can also be applied to investigate complex molecules and chemistry.
While studying the free-floating molecules in a gas using the uniquely bright X-rays at LCLS, a clear picture of the structural changes occurring in the chemical reaction can be observed as there is much less chance of the gas molecules becoming tangled or the view to be obstructed. "Until now, learning anything meaningful about such rapid molecular changes in a gas using other X-ray sources was very limited, at best," he added.
XPP | New ‘Molecular Movie’ Reveals Ultrafast Chemistry in Motion
This video describes how the Linac Coherent Light Source, an X-ray free-electron laser at SLAC National Accelerator Laboratory, provided the first direct measurements of how a ring-shaped gas molecule unravels in the millionths of a billionth of a second after it is split open by light. The measurements were compiled in sequence to form the basis for computer animations showing molecular motion. (Credit- SLAC National Accelerator Laboratory)
New Views of Chemistry in Action
The researchers used pine oil-derived 1,3-cyclohexadiene (CHD), small, ring-shaped organic molecules in its gas form for the study. Most of the biological and chemical processes involve ring-shaped molecules triggered by the breaking or formation of chemical bonds. The experiment captured the unfolding of the ringed molecule after the breakage of a bond between its two atoms, converting into a nearly linear molecule called hexatriene.
"There had been a long-standing question of how this molecule actually opens up. The atoms can take different paths and directions. Tracking this ultimately shows how chemical reactions are truly progressing, and will likely lead to improvements in theories and models," Minitti said.
The Making of a Molecular Movie
During the experiment, the researchers used ultrafast ultraviolet laser pulses to excite CHD vapor and initiate the ring-opening reaction. Then they investigated the structural changes of molecules using LCLS X-ray laser pulses at varying time intervals.
Researchers captured over 100,000 strobe-like measurements of X-rays that are scattered. The measurements were then compared with computer simulations that exhibit the possible ways in which the molecule unfolds in the first 200 quadrillionths of a second as soon as it opens. The simulations were carried out by Adam Kirrander, one of the team members from the University of Edinburgh, and they showed the changing position and motion of atoms.
The animations showed that each time interval indicates 25 quadrillionths of a second, which is faster than the typical 30-frames-per-second rate that display TV shows by about 1.3 trillion times.
"It is a remarkable achievement to watch molecular motions with such incredible time resolution," Weber said.
The study used a gas sample as it minimized any interference caused by neighboring CHD molecules, which makes it convenient to determine and capture the individual molecule transformation. Like the cue balls in billiards, the LCLS X-ray pulses scatter the electrons present in the molecules over a position-sensitive detector that identifies the location of atoms in the molecules.
A Successful Test Case for More Complex Studies
"This study can serve as a benchmark and springboard for larger molecules that can help us explore and understand even more complex and important chemistry," Minitti said.