Aug 11 2015
Researchers at Argonne National Laboratory have developed a copper tetramer catalyst that could help in capturing and converting carbon dioxide in a way that eventually saves energy.
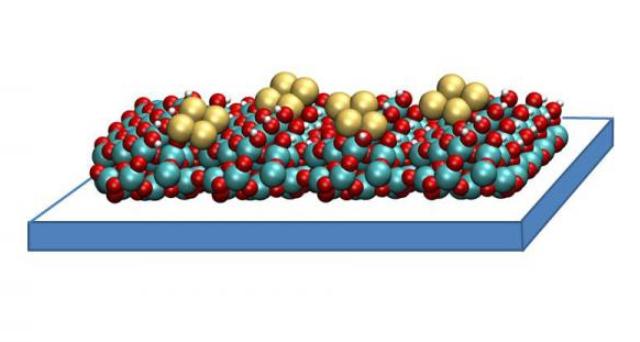 A copper tetramer catalyst created by researchers at Argonne National Laboratory may help capture and convert carbon dioxide in a way that ultimately saves energy. It consists of small clusters of four copper atoms each, supported on a thin film of aluminum oxide. These catalysts work by binding to carbon dioxide molecules, orienting them in a way that is ideal for chemical reactions. The structure of the copper tetramer is such that most of its binding sites are open, which means it can attach more strongly to carbon dioxide and can better accelerate the conversion. (Image courtesy Larry Curtiss)
A copper tetramer catalyst created by researchers at Argonne National Laboratory may help capture and convert carbon dioxide in a way that ultimately saves energy. It consists of small clusters of four copper atoms each, supported on a thin film of aluminum oxide. These catalysts work by binding to carbon dioxide molecules, orienting them in a way that is ideal for chemical reactions. The structure of the copper tetramer is such that most of its binding sites are open, which means it can attach more strongly to carbon dioxide and can better accelerate the conversion. (Image courtesy Larry Curtiss)
This innovative catalyst includes tiny clusters of four copper atoms that are supported on a thin aluminum oxide film. The catalysts function by adhering to the molecules of carbon dioxide and directing them in a manner suitable for chemical reactions.
The copper tetramer structure is designed in such a way that majority of its binding sites remain open, making the structure to bind more strongly to carbon dioxide and speed up the conversion process.
Carbon dioxide emission is a continuous environmental concern. Carbon dioxide reduction is based on the principle of capturing and converting - a process, which prevents the greenhouse gas before it emits from power plants and from chimneys and releases into the atmosphere and finally converts it into a practical end product.
Methanol is one potential end product which is a liquid fuel and has been the focus of a study carried out at Argonne National Laboratory, U.S. Department of Energy (DOE).
The chemical reactions which convert carbon dioxide into methanol mainly depend on a catalyst to accelerate the conversion process. Researchers at Argonne National Laboratory discovered a novel material that could prove suitable for this process. This catalyst features a special structure and can capture and change carbon dioxide in a way that saves energy. This catalyst was called as copper tetramer.
In the existing industrial process used for converting carbon dioxide to methanol, a catalyst made of zinc oxide, copper, and aluminum oxide is utilized. Several binding sites of the catalyst are occupied by simply holding the compound together which restricts the number of atoms that can catch and sustain carbon dioxide.
“With our catalyst, there is no inside,” said Stefan Vajda, co-author on the paper and senior chemist at Argonne and the Institute for Molecular Engineering. “All four copper atoms are participating because with only a few of them in the cluster, they are all exposed and able to bind.”
In order to offset a catalyst having fewer binding sites, the present technique of reduction generates high-pressure conditions to promote stronger bonds with the molecules of carbon dioxide. However, a large amount of energy is required to compress gas within a high-pressure mixture. A major advantage of improved binding is that the novel catalyst needs less energy and lower pressure to generate the same quantity of methanol.
The study authors stress that optimal ways should be identified to deal with the waste and reduce carbon dioxide emission.
“We’re interested in finding new catalytic reactions that will be more efficient than the current catalysts, especially in terms of saving energy,” said Larry Curtiss, an Argonne Distinguished Fellow who co-authored this paper.
Copper tetramers can make it possible to capture and transform carbon dioxide on a large-scale basis, reduce the global environmental problem, and produce a useful end product such as methanol that can be burned for fuel. However, this will take time to transform the catalyst from laboratory to industry.
Curtiss stated that possible barriers are instability and finding out a way to produce large quantities. However, copper tetramers could likely decompose in an industrial setting, and hence long-term durability is an important step for future studies. Although the researchers required only a small amount of material for their research, that amount would need to be increased considerably for industrial purposes. In the interim, the team is exploring other catalysts that can possibly surpass their copper tetramer. According to Vajda, such catalysts can differ in terms of composition, size and support material, resulting in over 2,000 possible combinations.
According to Peter Zapol, co-author of this paper and an Argonne physicist, the researchers would not have to perform different types of experiments, but instead they will utilize sophisticated computations to make predictions and then ultimately study the most promising catalysts.
“We haven’t yet found a catalyst better than the copper tetramer, but we hope to,” Vajda said. “With global warming becoming a bigger burden, it’s pressing that we keep trying to turn carbon dioxide emissions back into something useful.”
For the study, the researchers utilized the Center for Nanoscale Materials and also beamline 12-ID-C of the Advanced Photon Source, which are both DOE Office of Science User Facilities.
Curtiss added that the Advanced Photon Source enabled the team to view extremely low loadings of their small clusters, down to a few nanograms, which was a critical piece of this investigation.
The study titled “Carbon dioxide conversion to methanol over size-selected Cu4 clusters at low pressures,” appeared in the Journal of the American Chemical Society.
Co-authors also included researchers from the Yale University and the University of Freiburg. The DOE’s Office of Basic Energy Sciences funded the study.