May 5 2016
For the first time, researchers have successfully imaged all of the steps involved in a complicated organic reaction, and subsequently resolved the microscopic mechanisms that account for their behavior. The study was performed by an international team of scientists, including the UPV/EHU's Nano-Bio Spectroscopy Research Group.
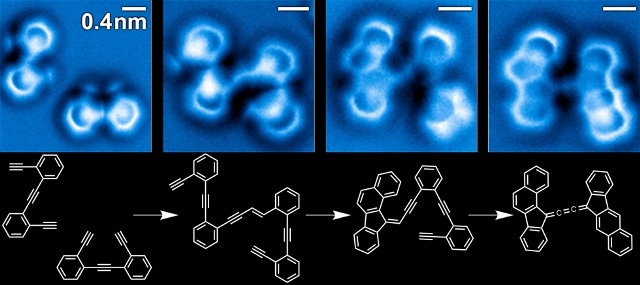 Sequence of images of the steps in the reaction of enediyne molecules on a silver surface (A. Riss / Technische Universität München).
Sequence of images of the steps in the reaction of enediyne molecules on a silver surface (A. Riss / Technische Universität München).
The research team has accurately detected the bond configuration of the intermediates, the reactants, and the final products in an intricate series of chemical changes of enediyne molecules on a silver surface at a single molecular level. The results of the study have been reported in the recent issue of the Nature Chemistry journal.
Chemists have been trying to track and directly view the way molecular structures change when they experience intricate chemical changes for a long time. Reaction intermediates are unstable substances that form in multiple steps during a reaction prior to obtaining the products. These intermediates have short lifetimes and are rather difficult to detect. If the structure of these intermediate species is known, it would be useful to understand the microscopic mechanisms of the reactions, and this may positively impact the materials science, chemical industry, materials science, biology, medicine, and nanotechnology fields.
The study was headed by Felix R. Fischer and Michael F. Crommie from the University of California at Berkeley and the Lawrence Berkeley National Laboratory, and by Angel Rubio, Professor at the UPV/EHU-University of the Basque Country, leader of the UPV/EHU's Nano-Bio Spectroscopy Research Group, and Director of the Max Planck Institute for the Structure and Dynamics of Matter in Hamburg.
A distinguished team of researchers imaged and resolved the bond configuration of the intermediate species, the reactants, and end products of an intricate organic reaction. Using non-contact atomic force microscopy (nc-AFM) with a highly sensitive tip, the researchers eventually acquired the images of the chemical structures related to varied steps of the reaction cascade that involves many steps of enediyne molecules on a silver surface. An extremely fine needle used by nc-AFM is capable of detecting even the tiniest bump on an atomic scale, just like reading in Braille, as the needle absorbs a molecule of carbon monoxide that serves like a "finger" on the text to improve its resolution.
[The accurate detection of the bond configuration of the intermediates] has made it possible to determine the intricate sequence of chemical transformations along the reaction mechanism from reactants via intermediates to end products, and at the same time unravel the microscopic mechanisms behind that intricate dynamical behavior.
Ángel Rubio, Professpr, UPV/EHU
Stabilizing the intermediates
Using a combination of traditional analytical models that illustrate the kinetics of sequential chemical reactions and the latest developments in numerical calculus, the researchers demonstrated an area that analyzes the molecular events and the rate of the reactions occurring in it. To better explain the stabilization of the intermediate species, the energy dissipation as well as the transformations in molecular entropy has to be considered, apart from accounting their potential energy. Molecular entropy determines the extent a system is organized.
The surface, especially the interaction of the unstable intermediate species with the surface contributes to the entropy and energy dissipation, thus emphasizing a basic difference between the solution or gas-phase chemistry and the surface-supported reactions.
[This level of comprehensive understanding acquired through the synergy between the latest developments in computer modeling and the imaging of a molecule’s chemical reactions] constitutes a fundamental milestone in the analysis of chemical reactions. Many of the limitations in conventional spectroscopic techniques have been surpassed and an unprecedented image has been obtained on an atomic scale of the reaction mechanisms, driving forces and kinetics.
Ángel Rubio, Professpr, UPV/EHU
Rubio added that all this newly found understanding could pave the way for an unlimited number of fields that have not been studied so far, such as materials science and biochemical applications, futuristic designs and optimizations of different catalytic systems, and fabrication of new synthetic tools for carbon-based nanotechnology.