May 31 2016
A team of researcher have developed a novel a metal-containing compound, which changes to a solid with exposure to light and on heating returns back to liquid form. The study was headed by Professor MOCHIDA Tomoyuki from Kobe University Graduate School of Science and Dr. FUNASAKO Yusuke from Tokyo University of Science, Yamaguchi.
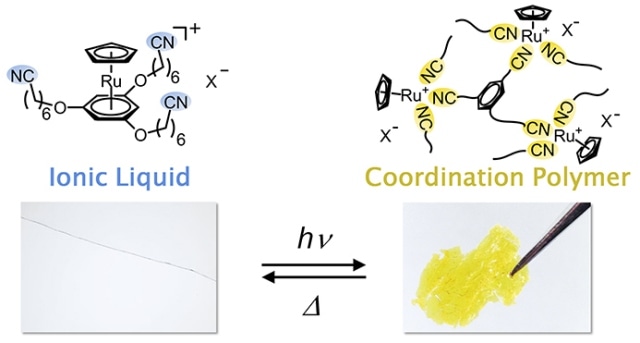 The chemical structure (above) and appearance (below) of the ionic liquid and coordination polymer. The ionic liquid is clear and colorless, but when exposed to ultraviolet light, the bonds between ruthenium and benzene rings are dissociated and replaced by a structure in which cyano groups link with the ruthenium ions, transforming it into a yellow solid. This solid reverts to the original liquid when exposed to heat. (Kobe University Graduate School of Science)
The chemical structure (above) and appearance (below) of the ionic liquid and coordination polymer. The ionic liquid is clear and colorless, but when exposed to ultraviolet light, the bonds between ruthenium and benzene rings are dissociated and replaced by a structure in which cyano groups link with the ruthenium ions, transforming it into a yellow solid. This solid reverts to the original liquid when exposed to heat. (Kobe University Graduate School of Science)
This compound could be used for various applications like photolithography technology where printed circuits are fabricated. This discovery was reported in the Chemical Communications journal.
Recently, there has been increased interest in the research of coordination polymers, which are solids with several useful applications. Researchers have created different methods to synthesize coordination polymers, however most of them depend on chemical reactions within solutions. For the first time, researchers have developed a method which forms coordination polymers from liquids on exposure to light.
The major factor to produce materials for electronic use is the application of techniques which can regulate the materials’ properties via external stimuli like heat and light. For instance, it is difficult to reuse the materials which solidify on exposure to light. These photosensitive resins are generally used for developing printed circuits.
The research team of Professor Mochida suggested that they could develop a material that could change its properties significantly on exposure to external stimuli if they could manage the binding process between organic molecules and metal ions by using light and heat. These researchers are the first to create an ionic liquid from a cyano group containing ruthenium complex. This ionic liquid is clear, colorless, non-volatile and also does not freeze at -50℃. On exposing the liquid to UV light for a few hours, it transforms into an amorphous coordination polymer. On heating this solid coordination polymer at 130℃ for a minute, it transforms back into its original ionic liquid form.
On application of heat and light in this manner, the researchers discovered a reversible transformation between a solid coordination polymer and an ionic liquid; both differing in their structures and chemical properties.
The researchers have successfully created a photocurable liquid, which is reusable. This liquid has potential application in 3D printing, printed circuit boards, and adhesives.
We plan to continue research on the molecular design of this substance, to reduce its response time, and look into creating more functions for this coordination polymer.
Professor Mochida Tomoyuki, Graduate School of Science, Kobe University