Feb 15 2017
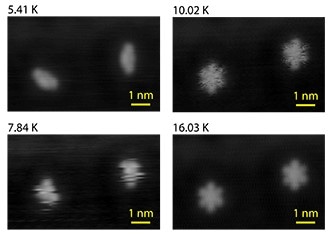 Images of a single molecule of dibutyl sulfide captured by a scanning-tunneling microscope (STM) at temperatures ranging from 5.41 degrees Kelvin (K) to 16.03 K. As the temperature increases, the molecule changes shape more quickly resulting in an image that captures multiple configurations of the molecule. The tip of the STM influences the ability of the molecule to make these changes in shape allowing researchers to measure the entropy of the system. Credit: J.C. Gehrig, EMPA.
Images of a single molecule of dibutyl sulfide captured by a scanning-tunneling microscope (STM) at temperatures ranging from 5.41 degrees Kelvin (K) to 16.03 K. As the temperature increases, the molecule changes shape more quickly resulting in an image that captures multiple configurations of the molecule. The tip of the STM influences the ability of the molecule to make these changes in shape allowing researchers to measure the entropy of the system. Credit: J.C. Gehrig, EMPA.
A recent study demonstrates that a scanning-tunneling microscope (STM), used to analyze changes that occur in a single molecule’s shape at the atomic scale, creates an impact on the molecule’s ability to carry out these changes.
This week’s issue of the journal Nature Communications has published a report on this study, demonstrating that the position of the tip of the STM relative to the molecule changes the molecule’s energy requirements in order to bring about changes in shape, thus changing the entropy of the system.
Entropy is often thought of as a measure of disorder or randomness, but here it is determined by the number of shapes that the molecule could potentially take, as well as by the number of different ways that the molecule could meet the energy requirements to change its configuration. If the tip of the STM increases the energy required by the molecule to make a change in shape, it is also increasing entropy in the system. In essence, a transition requires a potentially large number of small-energy excitations to co-occur to overcome the energy barrier for a configuration change. The larger the number of excitations required, the more ways in which those excitations may be collected. This multiplicity gives rise to entropy.
Eric Hudson, Associate Professor of Physics, Penn State
“That was totally unexpected,” said Hans Joseph Hug, professor of physics at EMPA, the Swiss Federal Laboratories for Materials Science and Technology and an author of the paper. “It meant that the tip -- which is still relatively far away from the molecule and in no-way touches it -- somehow influences the molecule’s mobility.”
The rate at which the single molecule “hops” between the shapes and the number of various other possible shapes that the molecule can take – a representation of the entropy of the molecule – changes based on the distance between the molecule and the tip of the STM.
“This means that the instrument we are using is affecting the system we are trying to study,” said Hudson. “But more importantly, it allows us to measure the molecule’s entropy and the fundamental relationship between entropy and the energy requirements of the molecule to make conformational changes.”
The researchers were focused on understanding what drives the ability of a molecule to modify its shape, which is a common requirement of biological processes and chemical reactions.
An STM, comprising of a very fine wire with a sharp tip that can be placed with sub-atomic precision, was used by the researchers to examine changes in the shape of a single molecule of dibutyl-sufide, which is a lengthy hydrocarbon with a central Sulfur atom, fixed to a flat gold surface.
Current travels between the surface and the tip of the STM and as the tip scans through the surface the STM identifies changes in that current as it travels over the molecule. These modifications in current are then used to develop an image of the molecule.
At extremely low temperatures -- just a few degrees above absolute zero (-273 degrees Celsius or zero degrees Kelvin) -- the molecule moves very slowly and the STM captures almost a still image of the molecule. But as we raise the temperature even just a few degrees, the molecule moves faster and the image from the STM will show the molecule in more than one conformation. It’s like taking a photograph of a moving object with a slow shutter speed.
Eric Hudson, Associate Professor of Physics, Penn State
To understand the physical parameters that control the ability of a molecule to change shape, the STM was used by the team to analyze the changes in the shape of the dibutyl sulfide molecule at temperatures ranging from about 5 to 15 degrees Kelvin.
Two physical parameters are normally used to explain how free to move a molecule on a surface. These parameters include the attempt rate, how often the molecule tries to initiate the movement; and the activation energy, the energy barrier the molecule will have to overcome to carry out the movement in question.
The researchers surprisingly noticed that the molecule’s energy barrier changed based on the position of the STM tip, even at similar temperatures.
Additionally, the researchers observed that the position of the STM created an impact on the attempt rate of the molecule that is related to the entropy of the molecule.
This implies that the energy and the entropy in this system are somehow linked at a fundamental level. What’s more, our results imply that entropy plays a decisive role for the dynamics of the molecule even at very low temperatures where a molecule’s degree of freedom, and thus its “configurational” entropy, are usually significantly reduced and entropy is considered to only play a minor role.
Hans Joseph Hug, Professor, EMPA
“In our study of dibutyl sulfide, the fascinating observation is that raising the hurdle for the molecule’s change in shape -- the energy barrier for the movement -- simultaneously provides it with a greater number of pathways to overcoming it -- an increase in entropy,” said EMPA physicist Miguel A. Marioni, an author of the paper. “These findings imply that our home-built STM is a perfect tool for studying a single molecule’s entropy in great detail.”
“To me the thing that is coolest, is that entropy is this fundamental thing that we all learn about in school, but it’s never something that you measure,” said Hudson. “When you go in the lab it just disappears somehow, so that fact that we were able to measure it, and that the entropic forces are comparable to the forces we typically measure was just mind-blowing.”
In addition to Hug, Marioni, and Hudson, the research team also included Jeffrey C. Gehrig, Marcos Penedo and Manfred Parschau, at Johannes Schwenk at EMPA. The Swiss National Science Foundation and EMPA funded the research.