Apr 5 2017
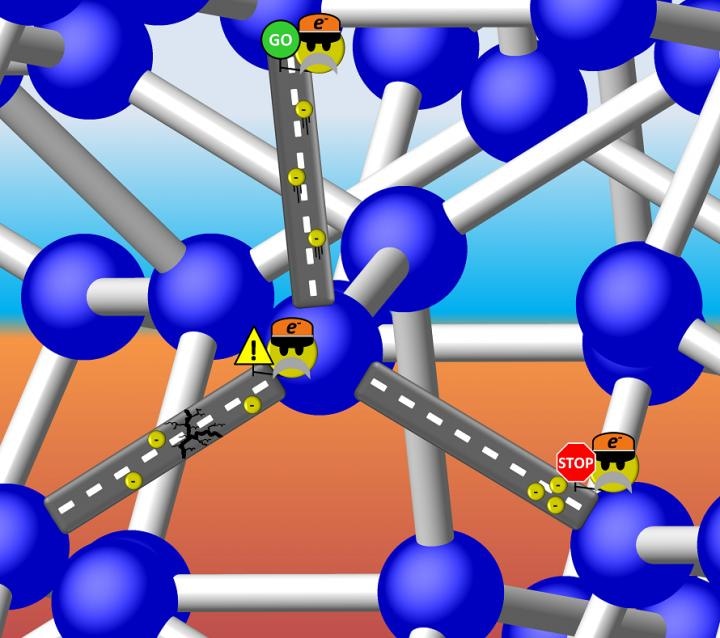 Is Si-III a metal with freely travelling electrons, or a semiconductor with a discrete energy gap that can "stop" the flow? It turns out the latter is true, but the band gap of Si-III is so small that electrons can "proceed with caution" through the structure. (Illustration is courtesy of Tim Strobel)
Is Si-III a metal with freely travelling electrons, or a semiconductor with a discrete energy gap that can "stop" the flow? It turns out the latter is true, but the band gap of Si-III is so small that electrons can "proceed with caution" through the structure. (Illustration is courtesy of Tim Strobel)
The importance of silicon cannot be overestimated especially with regards to solar energy, computing, and other technological applications. Element number 14 is also abundantly available in the Earth’s crust, and there is more to explore about how to harness its properties.
Silicon’s typical form crystallizes in the same structure as diamond. However, it is possible to create other forms using various processing methods.
A recent study led by Carnegie’s Tim Strobel demonstrates that one form of silicon, known as Si-III (or sometimes BC8), which is produced using a high-pressure process, is what’s referred to as a narrow band gap semiconductor. The details of the research have been published in Physical Review Letters.
What does this mean and why does it matter?
Metals are compounds capable of conducting electrons that constitutes an electric current, while insulators are compounds that cannot conduct any current at all. The electrical conductivity of semiconductors that are used widely in electronic circuitry can be turned on and off - a clearly valuable capability.
This capacity to switch conductivity is possible because a few of their electrons can travel from lower-energy insulating states to higher-energy conducting states when exposed to an input of energy. The energy required to trigger this leap is called a band gap.
Silicon’s diamond-like form is a semiconductor and other identified forms are metals, but the accurate properties of Si-III remained unidentified until now.
Earlier experimental and theoretical research submitted that Si-III was a poorly conducting metal without a band gap. However, so far, no research team could produce a pure and large enough sample to be certain.
Strobel and his team synthesized pure, bulk samples of Si-III, which helped them to establish that Si-III is truly a semiconductor with a very narrow band gap, narrower than the band gap of diamond-like silicon crystals, which is the most typical utilized version.
This indicates that Si-III could have applications over and above the already full array of applications for which silicon is presently used. Now with the availability of pure samples, the research team was able to totally distinguish the electronic, thermal, and optical transport properties of Si-III for the first time.
Historically, the correct recognition of germanium as a semiconductor instead of the metal it was once widely believed to be truly helped to start the modern semiconductor era; similarly, the discovery of semiconducting properties of Si-III might lead to unpredictable technological advancement. For example, the optical properties of Si-III in the infrared region are particularly interesting for future plasmonic applications.
Haidong Zhang, Carnegie
The paper’s other co-authors are Hanyu Liu, Zhenxian Liu, and Michael Guerette of Carnegie; Kaya Wei and George Nolas of University of South Florida; Oleksandr Kurakevych and Yann Le Godec of Institut de Minéralogie de Physique des Matériaux et de Cosmochimie; and Joshua Martin of the National Institutes of Standards and Technology.