Apr 18 2017
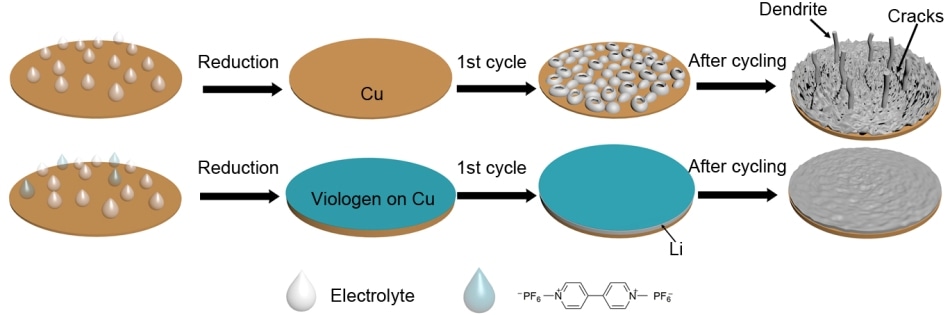 Illustrations of the design principles of using methyl viologen to form a stable coating to allow the stable cycling of lithium metal. (Credit: University of California, Riverside)
Illustrations of the design principles of using methyl viologen to form a stable coating to allow the stable cycling of lithium metal. (Credit: University of California, Riverside)
A key component of smartphones, laptops, and electric vehicles are high performing lithium-ion batteries. Currently, the anodes, or negative charged side of lithium ion batteries, are commonly manufactured with graphite or other carbon-based materials.
But, the performance of carbon-based materials is restricted because of the energy and weight density, which is the quantity of energy that can be stored in a specified space. Therefore, a lot of research is concentrated on lithium-metal anodes.
The success of lithium metal anodes will enable a number of battery technologies, including lithium air and lithium metal, which can possibly boost the capacity of the existing best lithium-ion batteries five to 10 times.
That would translate as five to 10 times more range for electric vehicles and smartphone batteries lasting five to 10 times longer. Lithium metal anodes are also less expensive and lighter.
The issue with lithium ion batteries made with metal is that during charge cycles they tend to uncontrollably grow dendrites, which are microscopic fibers that resemble tree sprouts.
The dendrites lower the performance of the battery and also pose a safety issue because they can short circuit the battery and in some cases even cause fire.
Researchers at the University of California, Riverside have made a noteworthy advancement in solving a more than four decade old dendrite problem. Their research findings were published in the journal Chemistry of Materials.
The team found out that by coating the battery with an organic compound known as methyl viologen they can stabilize battery performance, stop dendrite growth, and enhance the lifetime of the battery by more than three times compared to the present basic electrolyte used with lithium metal anodes.
This has the potential to change the future. It is low cost, easily manipulated and compatible with the current lithium ion battery industry.
Chao Wang, Adjunct Assistant Professor of Chemistry, UC Riverside
The team developed a new strategy to create a stable coating to improve the lifetime of lithium-metal anodes. They used methyl viologen, which has been used in other applications because of its ability to modify color when reduced.
The methyl viologen molecule can be dissolved in the electrolytes in the charged states. Once the molecules make contact with the lithium metal, they are instantly decreased to form a stable coating on top of the metal electrode.
By incorporating only 0.5% of viologen into the electrolyte, the cycling lifetime can already be improved by three times. Additionally, methyl viologen is inexpensive and can be easily scaled up.
The stable operation of lithium metal anodes, which the researchers have accomplished with the incorporation of methyl viologen, could push the development of next generation high-capacity batteries, including lithium air batteries and lithium metal batteries.
Wang warned that although the coating enhances battery performance, it is not a way to stop batteries from catching fire.
The research paper is titled: “In Situ Formation of Stable Interfacial Coating for High Performance Lithium Metal Anodes.” Besides Wang, the co-authors are: Hai P. Wu, Yue Cao, and Lin Geng, all of UC Riverside.
The UCR Office of Technology Commercialization has filed a patent application for the inventions above.