May 22 2017
Traditional chemistry is enormously powerful in relation to creating very varied and very complex microscopic chemical molecules. However, one thing remains out of reach - the synthesis of large structures up to the macroscopic scale, which would need incredible quantities of chemicals along with an elaborate and complicated method.
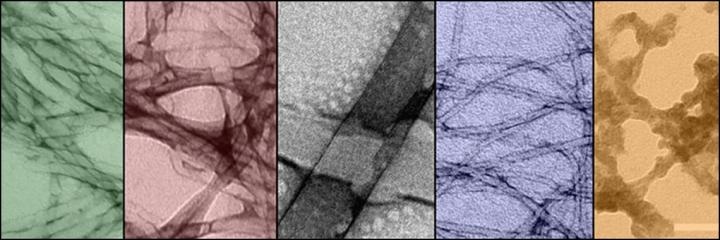 These are Transmission Electron Microscopy images of the self-assembling nanostructures, also visible to the naked eye. (CREDIT – OIST)
These are Transmission Electron Microscopy images of the self-assembling nanostructures, also visible to the naked eye. (CREDIT – OIST)
For this reason, researchers depend instead on “self-assembling” molecules, compounds that can relate with other copies of themselves to instinctively assemble into tubes, spheres, or other preferred shapes. Using this method, researchers at the Okinawa Institute of Science and Technology Graduate University (OIST) presently reports in Chemical Communications about new self-assembling molecules that can change into novel, exotic and formerly undetected shapes by just using UV light to force them to rearrange differently into “metastable” states.
When engineering self-assembly structures, researchers normally focus on the state of lowest energy – or “ground state,” in which the structure would be at its maximum stability. Less stable shapes are generally dismissed as undesirable and incorrect. However, this “ground state” being extremely stable makes it difficult to break down the structure if its shape has to be altered. In this research, OIST researchers inserted a weakness into their ground-state self-assembled structures, resulting in structures needing just a minor nudge to collapse. In this case, the nudge is the application of UV light to nick a specific bond between two atoms within the molecule, splitting the structure into smaller pieces. The pieces are then able to co-assemble into less stable – called metastable - but novel and unique shapes.
This report is about a new concept in material science. We converted a self-assembling phenomenon into co‑assembling in a spatially and temporally controllable manner using light. Eventually, we constructed exotic heterogeneous nanostructures inaccessible though conventional synthetic path.
Prof. Zhang from the Bioinspired Soft Matter Unit and the study’s author
This new concept paved the way to an interesting discovery: because the remaining fragments are closely packed following the collapse from the preliminary structure, they can form novel and unique structures which are not achievable if researchers were to simply mix the same molecules in free motion. To understand better, think of these nanostructures as Lego bricks: primarily there are just 2x5 bricks - five studs long and two studs wide– self-assembling into a nanofiber. UV light will cut these 2x5 bricks into two smaller pieces, for instance a 2x3 brick and a 2x2 brick, destroying the whole fiber-like structure. But since these smaller bricks remain pre-organized spatially remaining close to each other, they can effortlessly recombine themselves into new shapes visible to the naked eye. In contrast, if in a separate experiment researchers were to simply mix 2x3 and 2x2 Lego bricks in an arbitrary manner in a bucket with variable distances between bricks, their lack of spatial organization prevents the congregation of such novel nanostructures.
According to Prof. Zhang, the ability to develop new structures is important: “In material science, the function is always related to the structure. If you create a different structure, you manipulate the function and even create new applications. For example, the toxicity of a molecule in a nanofiber shape might be much lower or higher than the same molecule assembled in a spherical shape.”
The current research performed at OIST keenly recommends the preliminary conditions are the most important parameter impacting the final shape taken by self-assembling molecules. “If you know how the molecules pack with each other from the parameters of the initial state, then it will give you more clues to aim towards a specific macroscopic shape,” commented Prof. Zhang.
This shapeshifting capacity has immense potential for biological applications.
For example you introduce the molecule into a living organism and it adopts a certain structure. Then using light, you break a chemical bond and then the molecule will switch to another structure with the function you want.
Prof. Zhang
In pharmaceutical design, this sort of concept would allow a drug to reach its target in a living organism – a tumor or an organ– in an inactive state, thus controlling probable side effects. After it is broken down in this targeted location, the drug would reshape itself into a varied structure with therapeutic activity.
For now, using ultraviolet light as we do is not ideal as it is toxic for living cells. The next step for us is to move towards more biocompatible self-assembling structures with better adaptability to living systems.”
Prof. Zhang