This article covers how several detectors, especially light scattering detectors, can be used for size-exclusion chromatography (SEC) in protein analysis. SEC is extensively deployed for biopharmaceutical development in order to derive the component concentrations and molecular weight (Mw) of therapeutic proteins.
Molecular weight data is useful for identification and detection of oligomeric species and aggregates at the time of purity studies and to control the synthesis of advanced protein compounds. Precise molecular weight measurement of proteins is mandatory for assessing safety, activity and clinical efficacy.
Conventional SEC, which uses a single concentration detector, determines molecular weight with respect to a calibration standard instead of “absolute”. This is a specific problem for protein analysis, as unlike the standards, several proteins under study are not globular so the determined molecular weight will not be precise. However, light scattering detectors allow the measurement of absolute molecular weight without referring a calibration standard for all sample types.
This article studies how a light scattering detector can be added to a multi-detector SEC technique for consistent and robust measurements of molecular weight and for further insight into the behavior of proteins. The new study presented shows that light scattering detection can help remove inaccuracies, which can arise in traditional SEC due to non-uniform sample structures and conformations and sample-column interactions.
The Importance of Molecular Weight
Several aspects of the pharmacokinetics of a protein, like clinical efficacy or bioavailability, are based on molecular weight distribution and molecular weight. Accurate measurement of molecular weight is important for ensuring that biotherapeutic formulations regularly meet in vivo performance targets.
In certain cases, such as in forming active oligomer species, a molecular weight increase indicates successful protein manipulation. Careful tracking of oligomer molecular weight can show which protein state or assembly is the most effective. The key concern for the biopharmaceutical industry is inadvertent formation of protein aggregates, and it is mainly here that SEC is most suitable.
Studying how to control aggregation is important for offering product safety assurance, and for securing regulatory approval for commercializing new drug products.
Understanding How SEC Works
Based on hydrodynamic size, SEC separation is affected. A dissolved sample flows through a column packed with a microporous packing material, often an inert silica gel. It is observed that larger proteins diffuse into lesser number of pores in the packing material, and are the first to elute, whereas smaller proteins diffuse into a large number of the pores in the packaging material and have a higher elution time.
With conventional single-detector SEC, an external calibration curve is used for determining a relative molecular weight distribution from the retention and concentration time data recorded.
SEC can be used easily, and provides measurements of excellent repeatable measurements. However, there are a number of issues with single detector SEC.
For instance, results generated with SEC will not be consistent if:
- The reference standards used do not have the same hydrodynamic size or molecular weight relationship as the sample.
- Electrostatic interactions between the protein and column artificially extend elution time and compromise the reliability of analysis.
Furthermore, single detector analysis provides very little information about protein structure or conformation. The evolution of largely complicated biotherapeutic agents, and the associated need for "absolute" molecular weight measurements, have advanced SEC technology, including the use of a large number of detectors for measuring the size fractionated sample.
Advanced multi-detector SEC systems comprise a viscometer and a light scattering detector, alongside a traditional UV or RI concentration analysis for absolute Mw measurement without calibration, and a more comprehensive insight into protein structure. A direct measurement of absolute Mw is possible by adding a light scattering detector.
The interaction of a molecule with a light beam causes light scattering with intensity directly proportional to its molecular weight. Static light scattering (SLS) detectors determine intensity and convert these measurements to Mw data using the Rayleigh equation. These detectors are termed as right-angle, low-angle and multi-angle light scattering (RALS, LALS and MALS) detectors, based on the angle at which the scattered light is measured.
The Mw measurement obtained with light scattering detectors is not affected by retention time, without an external standard. Multi-detector SEC systems now also routinely have a viscometer for offering data on intrinsic viscosity or IV. Modifications in protein shape, structure and hydration, which lead to a change in the molecule’s mass to volume relationship, will result in an IV change.
The combination of absolute Mw and IV enables researchers to understand how this association and structure changes as a function of molecular weight distribution. It is possible to use this data to differentiate between oligomeric states, to determine the level of aggregation, and to observe shape or hydration parameters.
Application of Multi-detector SEC
These case studies show the value and application of multi-detector SEC for both enhancing the reliability of routine molecular weight calculations, and to provide precious insight into protein structure.
Case Study 1: Overcoming the Effects of Sample-Matrix Interactions
The first study involved using SEC for studying anthrolysin (ALO), a pore- forming cholesterol-dependent cytolysin secreted by Bacillus anthracis. According to research, the ALO protein is useful for anthrax pathogenesis, making it a significant protein target when studying the disease. Similar to other proteins, ALO shows electrostatic interaction with several column packing materials that can extend retention time artificially and effect the consistency of conventional or relative molecular weight measurements.
Experiment: With the aid of a multi-detector SEC system with a light scattering (LALS/RALS) detector, viscometer, and RI detector (Viscotek TDAmax, Malvern Panalytical), experiments were conducted. A Superdex 200 sample (GE Healthcare) in a buffer containing 20 mM Tris, 150 mM NaCl; pH 7.3 was used for the experiment.
Results: The chromatogram for the ALO monomer is shown in Figure 1. A retention volume of 22.2 mL was recorded. This corresponds to a 15-20 kDa molecular weight using conventional calibration techniques with the most relevant standard. The light scattering data shows that the weight-average molecular weight (Mw) of the ALO is around 53.6 kDa. This considerable underestimation of Mw cannot be easily detected without light scattering.
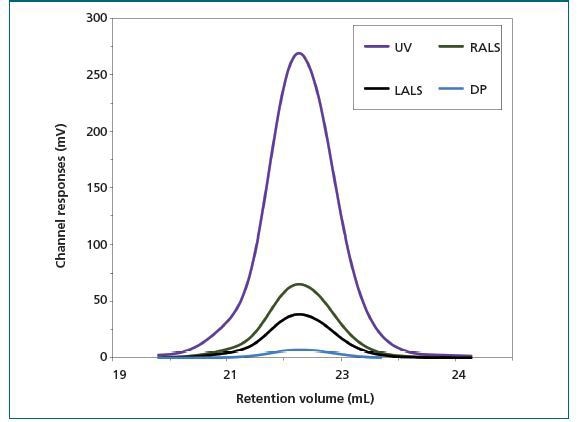
Figure 1. Chromatograms of anthrolysin (ALO) from a multi-detector SEC system showing traces from the UV (purple), RALS (green), and LALS (black) detectors; and the viscometer. Using these together enables accurate molecular weight determination and provides insight into protein structure. DP (blue) = differential pressure.
The concurrent IV measurements offer insight into the ALO monomer structure. Based on prior research, it was suggested that molecular density data can be used for estimating the shape and hydration of ALO.
The results obtained are:
- Mw of 53.6 kDa
- an IV of 5.1 mL/h
This suggests that the amount of hydration of the ALO is low, that the ALO monomer is elongated and the protein has a folded structure. This conclusion is supported by the conical shape observed in X-ray analysis of the protein structure. This insight is useful in the formulation of proteins to attain desirable activity in the finished product.
Case Study 2: Overcoming the Effect of Sample Structure on Sample Elution Volume
Several Bcl-2 proteins are molecular transducers, which show sensitivity to external and internal apoptic signals, playing an important role in apoptosis, or cell death regulation.
Protein aggregation results in amyloid fibre aggregation, which is related to several neurological degenerative conditions such as Alzheimer's and Parkinson's disease.
Using single detector SEC, tracking aggregation progression is difficult as there is an unpredictable variability in hydrodynamic volume and radius as mass increases.
In this study, experiments were conducted to analyze the in vitro formation of Bcl-2 aggregates after protein incubation at 37 °C using a light scattering detector to do away with the inaccuracies associated with conventional SEC.
Experiment: Using a Viscotek TDAmax (Malvern Panalytical), analysis of Superose 6HR (GE Healthcare) samples in a buffer of 20 mM sodium phosphate (pH 8) and 150 mM sodium chloride was performed. The aggregation process was examined after incubation for both one day and one week.
Results: After a one day incubation period, three key species were observed at retention volumes 22.6 mL, 23.6 mL, and 25.1 mL. Characterization with light scattering reveals that these peaks correspond to molecular weights of 74 kDa, 51 kDa, and 25 kDa respectively (Figure 2). The key sample population was found to be the latter species, which corresponds with the protein's monomeric state.
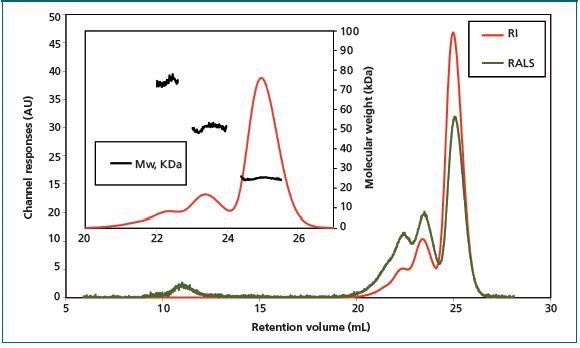
Figure 2. Chromatogram of Bcl-2 sample shows the presence of three different species after incubation at 37 °C for one day.
With the help of traditional column calibration, the retention time for the Bcl-2 monomer corresponds with an apparent Mw of 40 kDa. This implies that the Bcl-2 monomer’s hydrodynamic radius is larger than expected. Assuming a restricted interaction between the sample and column matrix, this impact could be attributed to high protein hydration or deviation from a spherical shape.
In fact, Bcl-2 is a globular protein having eight alpha helices and a flexible loop linking the first alpha helix to the core of the protein. This flexible loop plays a critical role in determining the elution profile of the sample, as it effectively gives the protein a larger hydrodynamic radius than would be expected for a compact protein with the same Mw.
After incubation for a week, incubation was more advanced. According to the results, the first step of the fiber growth is small aggregates being formed, mostly dimers and turners, which further assembled into larger aggregates. Multi-detector SEC consistently and clearly identifies these growing aggregates to offer useful insight into protein behavior.
Conclusion
This research shows the benefits of multi-detector SEC for protein analyzes. Using multiple detectors, and especially a light scattering detector, optimizes the informational productivity of SEC experiments. Using a light scattering detector along with a viscometer helps sense and monitor changes in molecular weight and structure in a manner not possible with a single detector system. The resulting data enhances the consistency of aggregate detection studies, and expands the use of the method in advanced biopharmaceutical research.

This information has been sourced, reviewed and adapted from materials provided by Malvern Panalytical.
For more information on this source, please visit Malvern Panalytical.