Accurate quantification of heavy metal ions is essential for many applications such as waste management, environmental monitoring, research studies, or some clinical tests. While heavy metals do occur naturally, increasing urbanization and industrialization over the past 200 years have resulted in increased levels of heavy metals in many environments.

Image Credit: Metrohm AG
As these hazardous elements are released they accumulate in soil, surface, or groundwater, potentially entering the food chain via drinking water or bioaccumulation in animals and plants. In fact, pregnant women are advised against eating seafood due to the potential for mercury (Hg) accumulation via the food chain.
The type of metal will determine its degree of toxicity, its biological role, and most crucially, its concentration. Raised concentrations of iron, cadmium, lead, copper, chromium, arsenic, or nickel in drinking water are the most common causes of human poisoning.
In order to highlight the toxicity of some heavy metals and to protect human health, organizations such as the World Health Organization (WHO), the U.S. Environmental Protection Agency (EPA) and the European Commission have each set limits or guideline values for the level of heavy metals in drinking water.

Image Credit: Metrohm AG
A number of techniques have been developed for heavy metal ion analysis previously. Commonly used techniques include inductively coupled plasma (ICP), atomic absorption spectrometry (AAS), or fluorescence spectrometry.
Each of these techniques requires expensive equipment, as well as extensive maintenance costs and highly trained personnel. Because of this, there is a clear need for a straightforward, cost-effective, and highly sensitive means of detecting metal ions in water samples.
Stripping voltammetry offers an ideal solution for these challenges, providing a rapid, simple, and inexpensive alternative to many other techniques. Stripping voltammetry is accessible to untrained personnel while being able to accommodate detection limits in the ng/L range. Furthermore, it can determine trace levels of heavy metals in the field, making stripping voltammetry an interesting, valuable method for mobile applications.
The Principle of Stripping Voltammetry
There are two steps involved in the voltammetric determination of heavy metals. The first step involves the analyte being preconcentrated on the working electrode’s surface, as illustrated in Figure 1 using the example of anodic stripping voltammetric determination of lead (Pb).
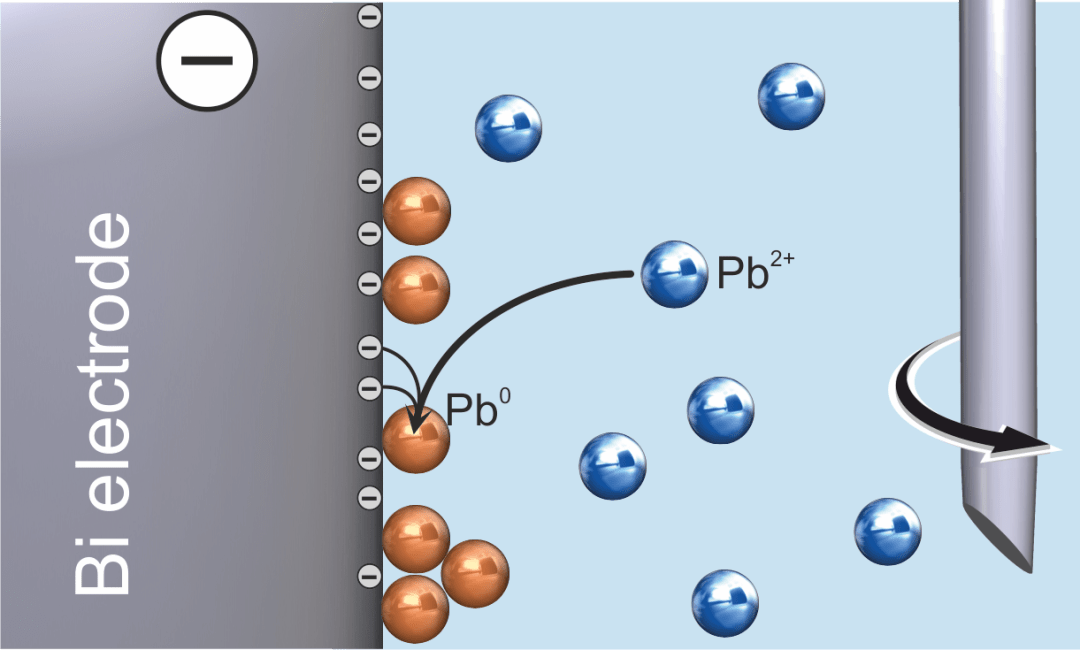
Figure 1. Anodic stripping voltammetry – deposition of lead (solution stirred). Image Credit: Metrohm AG
Step two is the stripping step (Figure 2), and it is here that the analyte is released via either reduction or oxidation, depending on the method used for determination. It is this step that generates the analytical signal, which must be proportional to the amount of analyte deposited.
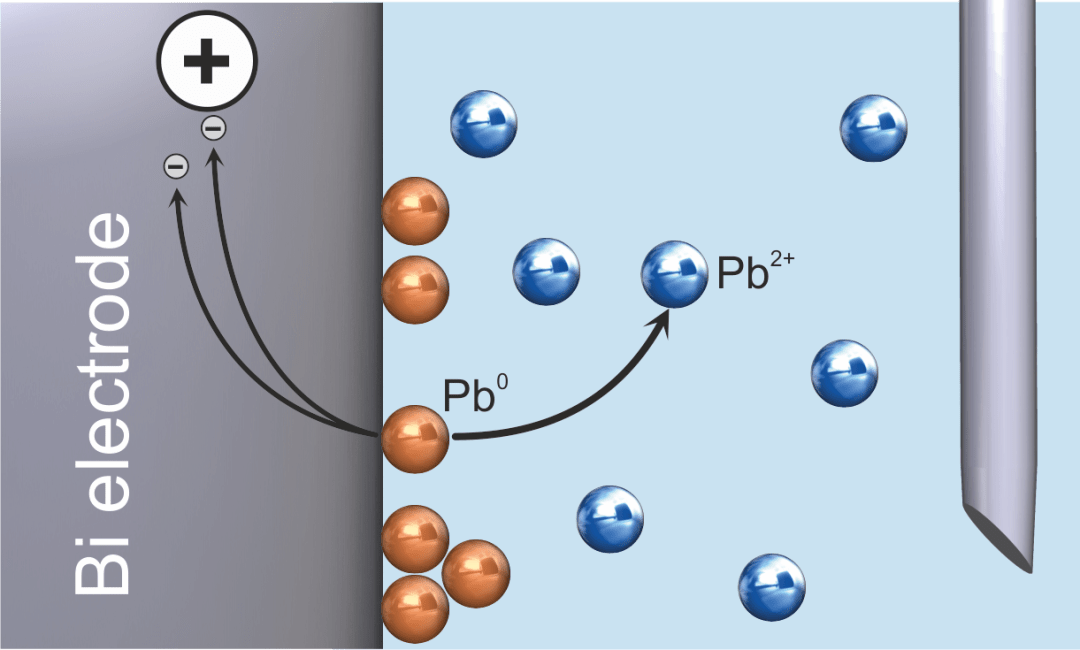
Figure 2. Anodic stripping voltammetry – stripping of lead (solution not stirred). Image Credit: Metrohm AG
As well as anodic stripping voltammetry, adsorptive stripping voltammetry or cathodic stripping voltammetry also function in a similar fashion.
Each of these different methods shares a common feature: a voltammetric determination will only be as good as the sensor employed in the measurement.
The Need for New Sensors
Research on new sensors in voltammetry tends to be triggered by environmental issues, sensor costs, and an ongoing need for heavy metal ion determination in the field.
Ideally, new sensors will be manufactured from inexpensive, non-toxic materials. These materials’ properties can lead to some restrictions, however. One issue is the limited number of elements that it is possible to detect on a certain electrode material like carbon, gold, or bismuth.
Successfully determining the number of elements simultaneously at the same mercury-free sensor can also be problematic, but this can be addressed through careful selection of the most appropriate electrode material along with an optimal sensor design.
Bismuth as an Alternative Electrode Material
There have been many attempts to locate less toxic electrode materials than mercury, suitable for the determination of heavy metal ions. Unfortunately, none of these materials have achieved a high level of electroanalytical performance. In the year 2000, an American researcher named Joseph Wang became the first person to report a bismuth film electrode.
Figure 3. Bismuth crystal. Image Credit: Metrohm AG
Following this revolutionary initial report, there has been a marked increase in the use of bismuth-based electrodes prepared as in-situ and ex-situ films on solid-state electrodes such as carbon.
Bismuth’s low toxicity and broad electrochemical window are key factors, as well as bismuth’s ability to form alloys with a high number of heavy metals. Bismuth also shows high hydrogen overpotential, similar to that of mercury.
These properties combine to make bismuth a particularly interesting option for stripping voltammetry. Hydrogen evolution is suppressed efficiently, meaning that noise-free measurements at negative potentials may be performed.
Bismuth electrodes based on bismuth films are a viable option, but the need for film deposition adds an extra, time-consuming step to the overall process.
New Sensor in VA: The Bi Drop Electrode
Thanks to the development of the Bi drop electrode, there is now a novel solid-state electrode available that is suitable for the determination of heavy metal ions in drinking water. A bismuth drop of around 2 mm diameter provides the working electrode within the voltammetric measurement.
The electrode can function without film deposition or polishing – it only requires electrochemical activation. This reduces the entire analysis time by a considerable amount and, once activated, series of highly repeatable heavy metal determinations in the low μg/L and even ng/L range become feasible.
The Bi drop electrode facilitates mercury-free monitoring of the limit values of the heavy metals lead, cobalt, cadmium, nickel, and iron in drinking water. Because the electrode does not necessitate mechanical treatment, it is particularly useful in online applications. A further benefit of the Bi drop electrode is the fact that lead and cadmium, or cobalt and nickel can be simultaneously determined.

Figure 4. Bismuth drop electrode from Metrohm. Image Credit: Metrohm AG
The sensor is stable, cost-effective, highly sensitive, and can provide more reproducible results than previously investigated bismuth-based electrodes.
In order to properly demonstrate the flexibility and range of possibilities offered by the Bi drop electrode, the following sections explore examples of anodic stripping voltammetry, adsorptive stripping voltammetry, and direct voltammetric determination.
Applications
Anodic Stripping Voltammetric Determination of Cadmium and Lead
The World Health Organization’s Guidelines for Drinking-water Quality set a maximum concentration of 3 µg/L for cadmium and 10 µg/L for lead in drinking water. These provisional guidelines aim to mitigate the toxic effects of cadmium on the kidneys, skeleton, and respiratory system, and the neurotoxic effects of lead.
The Bi drop electrode is mercury-free and is able to facilitate simultaneous determination of lead and cadmium in drinking water with no supplementary film plating step. A limit of detection (LOD) of 0.5 µg/L for lead and 0.1 µg/L for cadmium can be achieved with a 60 second deposition time - exceptional sensitivity which is more than sufficient to monitor the aforementioned provisional guideline values from the WHO.
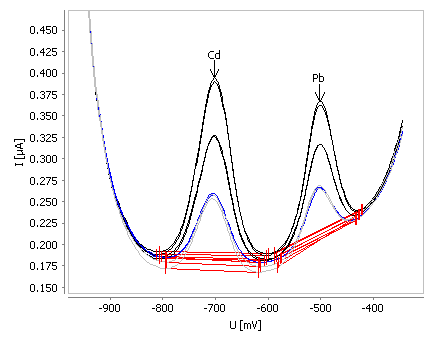
Figure 5. Example for determination of cadmium and lead in tap water spiked with β(Cd) = 2 µg/L and β(Pb) = 2 µg/L. Image Credit: Metrohm AG
This impressive sensitivity is coupled with accuracy and reproducibility. The relative standard deviation for 10 measurements in a check standard solution (β(Cd) = 1 µg/L and β(Pb) = 5 µg/L) is 5% and 3%, and the recovery rate is 100% and 90% for lead and cadmium, respectively.
Direct Determination of Iron
Iron present in drinking water may result in reddish-brown stains and an unpleasant, harsh metallic taste. Iron bacteria can grow in water containing as little as 100 µg/L iron, creating a reddish-brown slime that may cause an offensive odor or clog plumbing.
Insoluble iron deposits may form over long periods of time. This may cause ongoing problems in many agricultural and industrial applications, including system cooling, water supply, or field irrigation. In order to avoid these issues, the U.S. Environmental Protection Agency (EPA) has defined the Secondary Maximum Contaminant Level (SMCL) for water processing and treatment plants as 300 µg/L iron in drinking water.
There is no need for enrichment during the voltammetric determination of the iron triethanolamine complex on the non-toxic Bi drop electrode. The system utilizes catalytic signal enhancement, enabling detection at very low levels with a limit of detection of 5 µg/L, as well as measurements in a broad range of concentrations up to 500 µg/L.
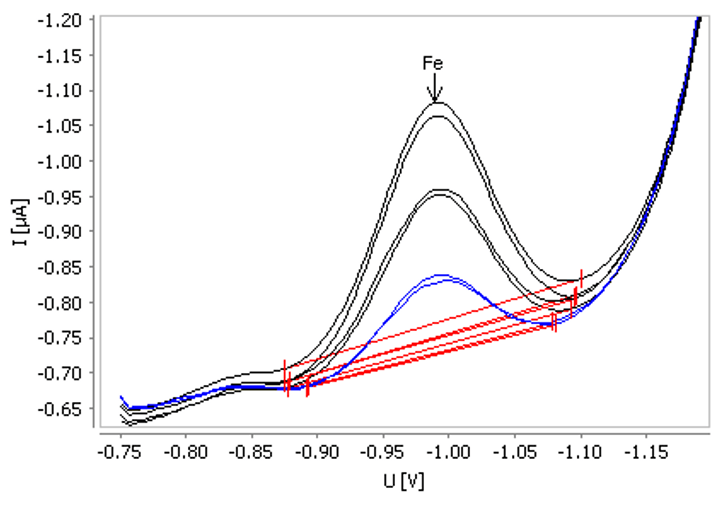
Figure 6. Example for determination of iron in tap water spiked with β(Fe) = 20 µg/L. Image Credit: Metrohm AG
This approach is ideal for process analyzers or automated systems, providing stable results and fully automated determination of iron in a large sample series. The relative standard deviation for a series of 10 measurements in a check standard solution (β(Fe) = 50 µg/L) is 3% while the recovery rate is 111%.
Adsorptive Stripping Voltammetric Determination of Nickel and Cobalt
Metallurgical operations, electroplating processes, and leaching from fittings and pipes are all primary sources of nickel pollutions. Catalysts employed in the chemical and petroleum industries are key application fields for cobalt.
Metal from each of these sources is either released directly or through the wastewater-river pathway and into the drinking water system. Because of this, EU legislation stipulates 20 µg/L as the limit value for nickel concentration in drinking water.
Adsorptive stripping voltammetry (AdSV) forms the basis of simple, simultaneous determination of cobalt and nickel.
The non-toxic Bi drop electrode’s unique properties in conjunction with AdSV results in excellent sensitivity and performance. The limit of detection for a 30 second deposition time is approximately 0.1 µg/L for cobalt and 0.2 µg/L for nickel. This can be further lowered via an increase in deposition time.
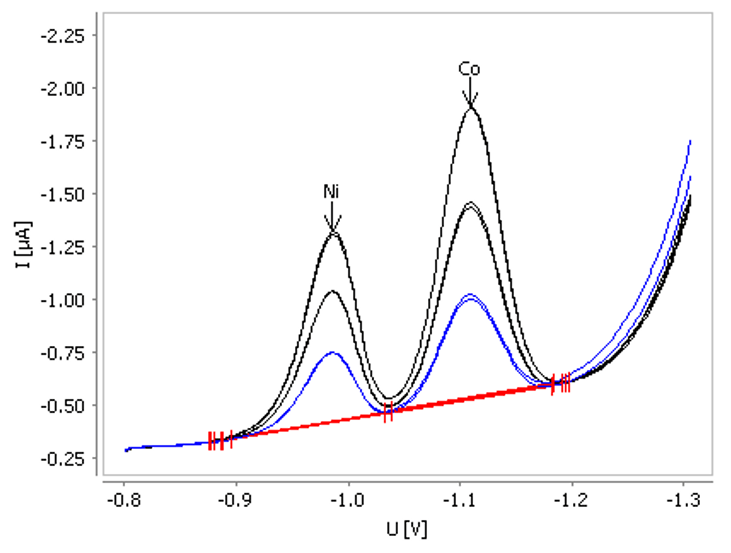
Figure 7. Determination of nickel and cobalt in tap water spiked with β(Ni) = 0.5 µg/L and β(Co) = 0.5 µg/L. Image Credit: Metrohm AG
This approach is best suited for automated systems or process analyzers, enabling automatic determination of these metals in large sample series, as well as providing accurate, stable results.
Relative standard deviation for 10 successive measurements in a check standard solution (β(Ni) = 1 µg/L β(Co) = 1 µg/L) is 4% and 5%. The recovery rate is 88% for cobalt and 106% for nickel.
Key Features of the Bi Drop Electrode
In summary, the Bi drop electrode offers the following key features:
- Non-toxic, mercury-free alternative ideal for trace metal determination
- Simultaneous determination of Co and Ni, as well as Pb and Cd
- Limit of detection in the low μg/L and ng/L range
- Ideal for online and automated systems
Acknowledgments
Produced from materials originally authored by Alyson Lanciki from Metrohm.

This information has been sourced, reviewed and adapted from materials provided by Metrohm AG.
For more information on this source, please visit Metrohm AG.