Metrohm’s Approach to Analytical Instrument Qualification (AIQ)

Image Credit: Metrohm AG
Metrohm’s response to Analytical Instrument Qualification is contained within the Metrohm Compliance Services document, while the most detailed level of documentation offered for AIQ is the IQ/OQ.
Metrohm’s IQ/OQ documentation provides the necessary documentation in close alliance with the main regulations from the USP, FDA, GAMP and PIC/S. This enables good documentation of Metrohm instruments’ suitability for almost any lab’s intended use.

Image Credit: Metrohm AG
Metrohm’s test procedures can prove that the hardware and software components function appropriately, both individually and as part of an entire system. With Metrohm’s IQ/OQ, users are optimally supported for the integration of its systems into existing current processes.
Metrohm’s high quality documentation means labs and users are always ‘audit ready.’
The Flexibility of a Modular Document Structure
Depending on the specific demands and work environment, Metrohm can offer a custom qualification approach by virtue of documentation modularity. If a lower level of qualification is required, only the necessary modules can be provided.
Metrohm’s documentation is made up of various modules, each of which documents the identity of the qualification reviewer alongside the Metrohm representative, as well as the document involved in the qualification, details of each instrument, and software.
This means that each module is independent, which ensures both full traceability and reliability of the system setup.
Cost-effective Qualification from Metrohm
Metrohm supports labs by applying a cost-effective qualification process, depending upon the requirements and the modules required. In effect, a qualification is not about carrying out unnecessary actions, it is about finishing the work required.
The risk assessment analysis determines the level of qualification necessary and on this basis, Metrohm focuses on testing only that what needs to be tested. If a device is transferred to another lab, it is key to consider which qualification steps (DQ, IQ, OQ, PQ) are really necessary in order to satisfy needs.
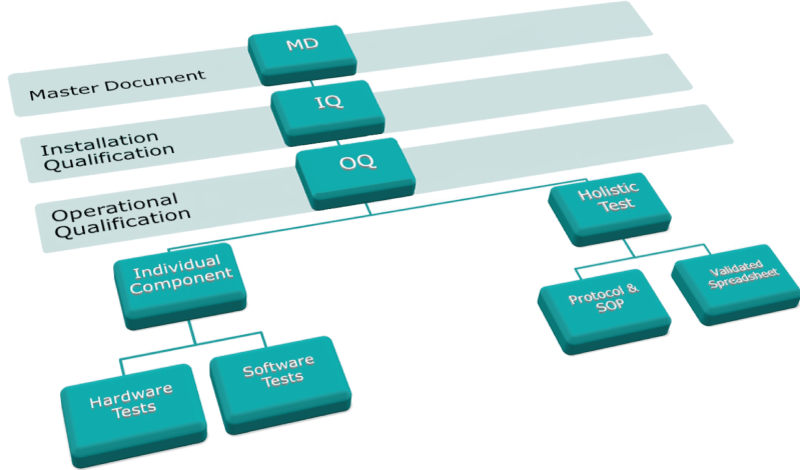
Image Credit: Metrohm AG
Metrohm can provide expert advice on this matter.
Metrohm IQ/OQ documentation was founded on the following documentation tree, starting with the first module, the Master Document (MD), followed by the Installation Qualification (IQ) and finally the Operational Qualification (OQ).
The OQ is then sub-divided into individual component tests (Hardware and Software) and a holistic test to verify the complete system.
Master Document (MD)
The Master Document (MD) is the start and central organizing document for the AIQ procedures of each qualification. It not only details the installation process and qualification of the instruments but also the education level and competence of the qualifying engineer.
The MD determines all other components to tally up to the qualification, leading to a flexible framework on which to develop a set of documentation.
Installation Qualification (IQ)
One definition of the documentation content is complete in the MD, the Installation Qualification (IQ) follows. This set of documentation was developed to ensure adequate delivery and installation of the instrument, software and any accessories have been completed.
Additionally, the IQ protocol details that the workplace is appropriate for the analytical system as stipulated by Metrohm.
Operational Qualification (OQ)
The main part of the qualification comes after proper installation has been completed: the Operational Qualification (OQ). In the first part of the OQ, testing and evaluation of the functionality of the single hardware components is performed to a set of procedures.
This is to make sure that the instrument is working as optimally designed and is safe for use. The expertise of Metrohm certified engineers to conduct these comprehensive tests on instruments using the necessary calibrated and certified tools should instill confidence.

Image Credit: Metrohm AG
The second part of the OQ is comprised of a set of Software Tests to verify that the installed Metrohm software works reliably and correctly on the computer it was installed on.
The significance of maintaining software in a validated state is also relative to the data integrity of the laboratory. These software tests can then be repeated periodically or after considerable changes.
In essence, these functionality tests cover verifications on audit trail review, backups, database functionalities, electronic signatures, security policy, user management and so on.
Metrohm constantly strives to improve its procedures and use innovative tools and technologies. For this reason, the company has implemented a test procedure that is totally automated for verifying the software of its new OMNIS platform.
This guarantees complete integrity in the execution and delivery of consistent results with rapid, error-free test execution.
This state-of-the-art, automated software validation gets rid of manual activities that are time consuming and labor intensive; this speeds-up testing and eliminates the inefficiencies that afflict the paper-based software validation.
The advantages are clear: reduce unnecessary laboratory startup activities and save valuable time during qualification, time that can be spent on other work in the lab.
Holistic Test (Performance Verification, PV)
Once testing of each individual component has been conducted, the entire performance of the system is proven by way of a holistic test (OQ-PV).
This is comprised of a series of ‘wet chemical’ tests, conducted utilizing certified reference materials, to validate systems is the capability to generate quality data, i.e., results that are precise, accurate and above all fit for the intended purpose.
On the basis of detailed, predefined instructions (SOPs), a series of conventional measurements are carried out, statistically evaluated and weighed up against the manufacturer’s specifications.

Image Credit: Metrohm AG
Differences Between Performance Verification (PV) and Performance Qualification (PQ)
Metrohm offers a set of tests known as the Performance Verification (PV) in order to confirm the instrument’s fitness for purpose. As previously mentioned, the PV is made up of standardized test procedures to guarantee optimal system operation in accordance with the manufacturer’s design in the selected environment.
On the other side, the Performance Qualification (PQ) qualification phase is extremely customer-specific (see the ‘4Q’s’ Qualification Phases in Part 1).
PQ authenticates the fitness for the purpose of the instrument under in-situ conditions of use, demonstrating its continued suitability. It can be said, PQ tests are determined in line with the specific analysis and acceptance criteria.
Further questions to consider—is the analytical instrument qualified for its intended use? Is the lab in compliance with the latest regulations for equipment qualification and validation? For expert advice, contact a local Metrohm agency and request a quote for Metrohm qualification services today.

This information has been sourced, reviewed and adapted from materials provided by Metrohm AG.
For more information on this source, please visit Metrohm AG.