AZoMaterials speaks to Dr. Simon Bates and Dr. Angela Criswell about how X-ray CT imaging is transforming tablet design and quality control, revealing how microstructure drives dissolution, performance, and regulatory confidence.
Why does tablet microstructure matter?
Simon: Two reasons. First, patient outcomes. Disintegration and dissolution control how the active ingredient is released and becomes bioavailable. If a tablet behaves like a brick, it may pass through undissolved; if it blows apart too fast, you can dump the API in the gut and lose bio-availability through recrystallization. All of these disintegration and dissolution behaviors are closely related to the tablet microstructure (Sun, Pharm. Res. 2016).
Second, quality and regulatory risk. Lots are released based on functional tests (like disintegration and dissolution). When a lot fails and you can’t identify the root cause, the whole process gets held up. It is an expensive, common problem; the root cause is often microstructure.
Microstructure (density, porosity, crack networks, and connectivity) drives these functional results, and varies with tablet press wear, powder flowability, and pressure. CT lets you measure and define an acceptable “microstructure envelope” that correlates with passing release tests. This is classic Quality by Design (QbD) and statistical process control. Establishing this envelope in the design process will make identifying the root cause much easier.
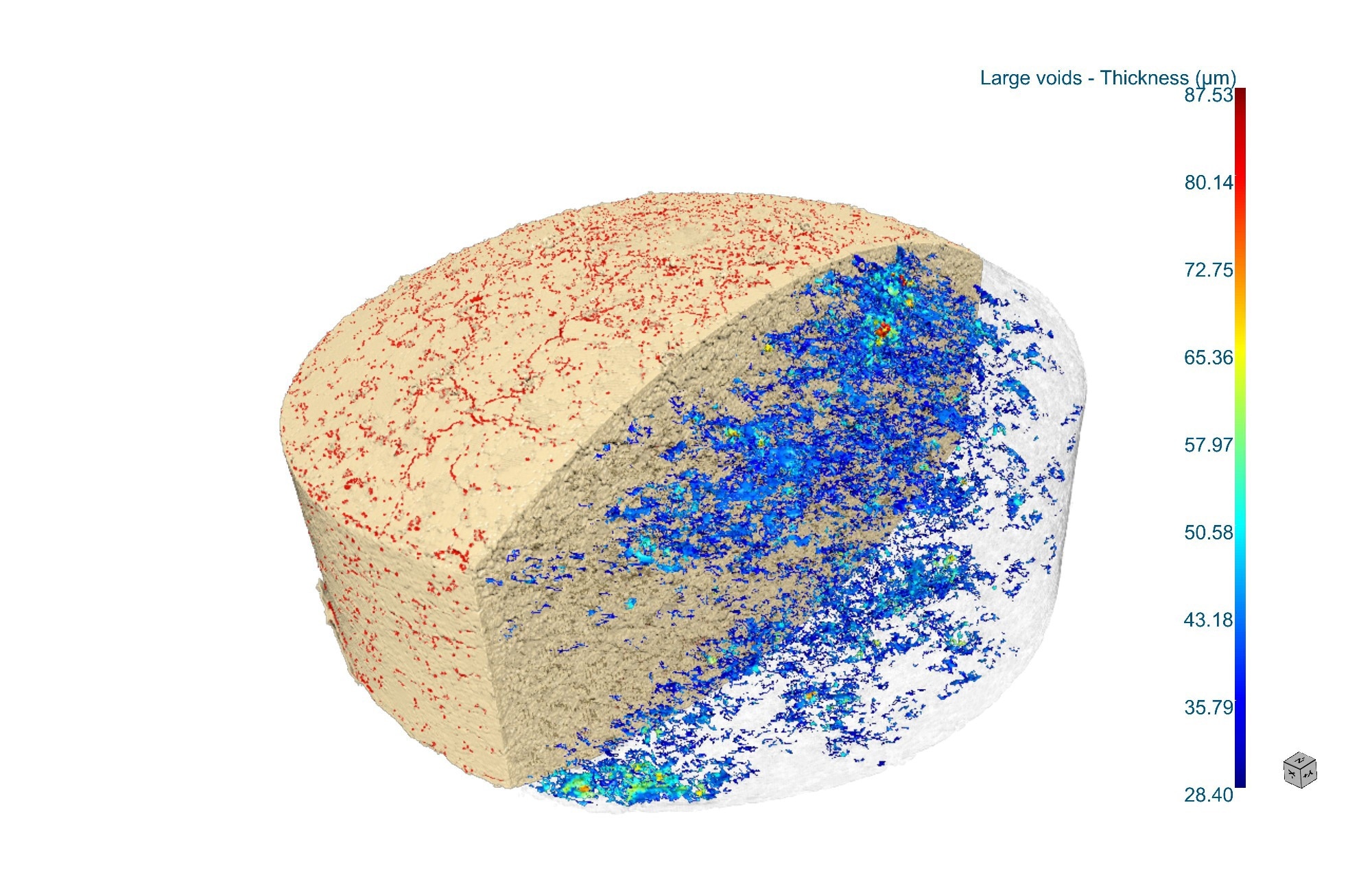
Thickness distribution analysis of pores and microcracks in a pressed tablet. Image Credit: Rigaku Corporation
What can CT resolve, and how long does it take?
Angela: CT displays the 3D material distribution of the tablets in grayscale, so air-filled pores and cracks have a strong contrast against the solid part of the tablets. You can also often discriminate excipient phases when the density differs. On resolution, cutting-edge absorption CT can push to ~100 to 200 nm or even ~50 nm with specialized X-ray microscopes or synchrotron radiation. Routine, affordable micro-CT scanners can resolve features at approximately five micrometers.
For tablet evaluation, a fast, practical scan at ~10 µm voxel resolves ~30 to 50 µm pores, cracks, and the connected pore pathways relevant to disintegration. If you need tighter, quantitative measurements on smaller features, plan for one to two hours to achieve approximately five-micrometers voxel resolution.
How do you navigate the trade-offs among resolution, field-of-view, and scan time?
Angela: It’s a three-way trade-off. A bigger field-of-view (entire tablet) at a higher resolution means longer scans. For QC screening, you often care about relative changes and “gross” defects, so a short (~15-20 minute) scan at ~10 µm voxels usually suffices.
For R&D quantitation, opt for longer one to two-hour scans to confidently evaluate cracks and pores ~10 µm and below. You can also employ deep-learning reconstruction and data enhancement techniques to increase the resolution without increasing the scan time.
At which decision points in early formulation can microstructure data change your choice, and why?
Simon: The industry widely accepts the microstructure-disintegration/dissolution link, but most groups still deploy CT late, at the production and release testing stage. To establish and police an acceptable microstructure window, however, the opportunity is earlier.
During excipient selection and compaction design, you can target connected mesopore/crack networks (≈10 to 50 µm) that allow dissolution media to reach disintegrants. Today, few formulators explicitly “design for microstructure,” but they should because microstructure is what the dissolution medium “sees.”

Dr. Angela Criswell, Director of X-ray Imaging, Materials Application Division. Image Credit: Rigaku Corporation
How do you approach reproducibility of microstructure analysis results across analysts and batches?
Angela: Treat CT like any regulated analytical method. Put formal IQ/OQ/PQ in place, define pass/fail rules, and automate segmentation for “air vs solid” to compute total porosity, for example. Focus on relative comparability for QC, such as “the same as yesterday and within the approved window,” and standardize and automate the workflow so operators can load samples and press start.
How do you validate CT-derived microstructure analysis metrics, such as porosity?
Angela: Use traceable standards with known feature sizes to establish what your system can and cannot see, and verify you cover the expected size range of pores and voids.
Simon: Then, correlate CT metrics to function. Run tablets through disintegration/dissolution and show predictive correlation to your CT-derived porosity features. Absolute porosity is important for validating a CT scanner and for system suitability testing, but ultimately, the release-test correlation is what operations care about.
How can people select the right CT system for tablets?
Angela: It depends on your needs. For example, a benchtop micro-CT is sufficient if you need QC screening of 30 to 50 µm cracks/connected pores in approximately 15 to 20 minutes. These generally include a ~130 kV source, flat-panel detector, and standard rotation stage, and they don’t need any special power supply, chiller, or room.
If you need sub-10 µm quantification, sub-micron features, or advanced phase contrast, expect higher cost and infrastructure.
Software also plays an important role. You want it to provide reliable air/solid segmentation and porosity in an automated workflow. There are various tools with different capabilities and price points. I would recommend testing the software to ensure you can obtain the desired results.
Simon: For at-line QC, you should consider sample handling for high-throughput measurements, including carousels or robotic loading.
Dr. Simon Bates, VP of Science and Technology. Image Credit: Rigaku Corporation
How does CT microstructure analysis plug into AI-driven formulation and materials discovery?
Simon: In AI-driven development, CT is a characterization node in a closed-loop “self-driving lab.” For each test tablet, CT can provide structural data, such as porosity, void distribution, or pore networks, which then become features for the AI planning agent.
The agent can use those CT-derived descriptors to choose the next formulation or process (excipient ratio, granulation route, compaction profile), allowing us to explore the composition–process space efficiently instead of conducting brute-force trials. The result is to set the structure targets and formulation parameters for acceptable disintegration/dissolution.
Another emerging technology is 3D printing multi-component tablets, where APIs/excipients are spatially patterned to precisely control the disintegration and dissolution process. These 3D-printed structures can even be tailored to individuals. CT is indispensable to characterizing these intricate tablet structures.
If one wants to introduce CT next quarter, what first steps would you recommend to start the process towards generating decision-ready microstructure data?
Angela: First, define the decision criteria, i.e., what you’ll accept/reject based on CT data, such as total porosity range, presence of connected voids above X µm, through-thickness porosity gradient. Then, assemble representative samples. Include “good,” “borderline,” and “bad” tablets that reflect your real variation.
Find a CT vendor or CRO (contract research organization) and run a pilot. You can send the set of representative samples for data collection and analysis. You can also request both the fast-screening recipe (~10 µm voxels, ~15 to 20 minutes) and a high-resolution recipe (~5 µm voxels, hours) with a completed analysis report.
I also recommend comparing at least two providers, as you are evaluating not just the tool but also the expertise of the provider to support you in the method development process.
Simon: If you are not sure you are ready to purchase a CT scanner, you can start by submitting tablets to a CRO. Once demand for CT testing increases, you can then consider bringing a system in-house.
About the Speakers

Dr. Simon Bates leads global business development at Rigaku, where he bridges analytical instrumentation with real-world R&D needs across pharma and other materials-intensive industries. His career spans comprehensive materials characterization. Simon also serves as an Adjunct Professor at Long Island University’s College of Pharmacy, teaching solid-state characterization, statistics, and data analysis. Prior roles include Research Fellow at Triclinic Labs and Principal at Aptuit Consulting. He holds a B.Sc. and Ph.D. in Applied Physics from the University of Hull.

Dr. Angela Criswell leads X-ray imaging at the Rigaku Americas division, specializing in 3D X-ray computed tomography (CT) and related X-ray techniques spanning diffraction, scattering, and crystallography. Over more than two decades with Rigaku, she has held roles in materials analysis and small-angle scattering product management, and is known for practical training and webinars that translate CT fundamentals into robust workflows. Angela earned a Ph.D. in Biochemistry & Cell Biology from Rice University and a B.S. in Biology from the University of Houston.

This information has been sourced, reviewed, and adapted from materials provided by Rigaku Corporation.
For more information on this source, please visit Rigaku Corporation.
Disclaimer: The views expressed here are those of the interviewee and do not necessarily represent the views of AZoM.com Limited (T/A) AZoNetwork, the owner and operator of this website. This disclaimer forms part of the Terms and Conditions of use of this website.