Sponsored by ELTRA GmbHReviewed by Emily MageeFeb 17 2026
Elemental analysis is central to mining operations and plays a role throughout the entire value chain, from early exploration and ore processing to process monitoring and quality control of the final product.

Image Credit: Shutterstock.com/New Africa
The assessment of material quality, key process decisions, and adherence to regulatory requirements all rely on reliable data on elemental composition.
There is often significant compositional variability in mined materials such as ores, concentrates, and fuels. As well as major elements, light elements such as oxygen, carbon, sulfur, and hydrogen also influence material behavior during thermal treatment, roasting, smelting, and combustion.
The presence of these elements impacts energy demand, reaction pathways, and process stability. Mining laboratories employ elemental analysis and loss-on-ignition (LOI) determination as standard analytical tasks for this reason.
LOI analysis delivers useful information on carbonates, moisture, hydrated phases, and other volatile components; elemental analyzers provide quantitative data on sulfur, carbon, and other non-metallic elements.
The combination of these methods supports routine material characterization and process control across a number of mining and mineral processing stages.
ELTRA GmbH has a range of elemental analyzers and thermal analysis systems for laboratories in mining and related industries. This article examines a series of common analytical applications, citing examples from copper, gold, iron ore, and coal mining.
Elemental Analysis Applications in Key Mining Sectors
Copper Mining: Carbon and Sulfur Analysis in Copper Concentrates
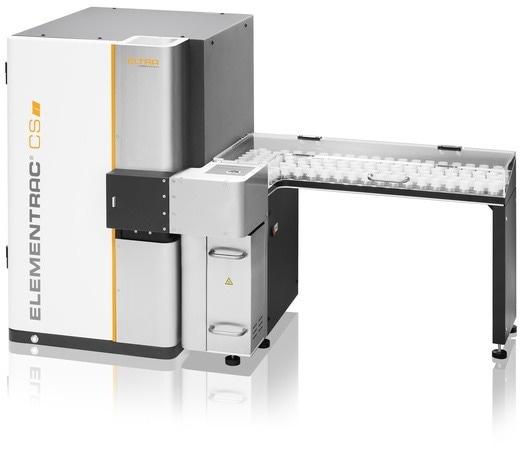
Image Credit: ELTRA
Copper concentrates typically contain increased sulfur levels due to sulfide mineralization. Carbon determination is also an important consideration, especially in terms of metallurgical process control.
Both of these parameters are monitored routinely due to their effect on gas composition, smelting conditions, and energy consumption.
The ELEMENTRAC CS-i uses a typical sample mass of 200 mg, operating in the extended four-cell configuration to enable carbon determination up to 35 % and sulfur determination up to 32 %. The ELEMENTRAC CS-i also covers low ppm concentrations.
This measuring range allows the analysis of concentrates with varying compositions with no need to alter the analytical setup.
Induction furnace combustion is used for carbon and sulfur determination, with infrared detection used to analyze CO2 and SO2.
This method can be used when working with concentrates containing either multi-percent sulfur or low carbon concentrations derived from carbon-bearing gangue minerals and flotation reagents.
ELEMENTRAC CS-i can be equipped with an autoloader for up to 130 samples, making it ideal for laboratories processing large sample volumes. This autoloader enables consistent throughput and unattended operation in routine quality control environments.
Gold Mining: Sulfur Screening in Exploration
Carbon / Sulfur Analyzer ELEMENTRAC CS-i - Introduction | ELTRA
Video Credit: ELTRA
Elemental analysis is regularly applied in gold exploration, allowing gold concentrations to be determined with confidence.
Sulfur is a useful indicator element during early exploration and resource evaluation because many gold deposits are associated with sulfide mineralization.
Sulfur screening can be used to identify sulfide-rich zones within host rocks, enabling the interpretation of mineralization patterns.
Variations in mineralization intensity are often highlighted by changes in sulfur concentration along drill cores or across surface samples, potentially indicating and supporting the prioritization of additional investigation.
The ELEMENTRAC CS-i r uses induction combustion with infrared detection of SO2 to facilitate this type of screening.
Sulfur can be determined from low ppm levels up to around 32 % with the extended four-cell configuration and a typical geological sample mass of 200 mg. This range is sufficient to accommodate both weakly mineralized material and samples that contain high sulfide content.
Short analysis times enable the processing of large sample series, support early-stage decision-making, and deliver consistent sulfur data for exploration programs.
Coal Mining: Carbon and Sulfur Determination with ELEMENTRAC CS-r

Image Credit: ELTRA
Carbon and sulfur analysis are key to effective coal characterization, as both parameters affect calorific value, emissions, and combustion behavior.
This data is an important factor in coal classification, environmental reporting, and pricing.
The ELEMENTRAC CS-r is designed for carbon-rich materials analysis, such as coal and graphite. Combustion is performed in a resistance-heated ceramic furnace, with infrared detection of CO2 and SO2. This approach ensures total oxidation, including refractory or high-carbon materials.
Carbon can be determined from low ppm levels up to 100 % in the four-cell configuration, using a nominal sample mass of 250 mg. It is also possible to measure sulfur up to approximately 44 %.
This enables trace analysis and high-content determination without requiring modifications to the system configuration.
Infrared detector paths are manufactured from solid-gold components, reducing the effects of aggressive combustion gases and contributing to stable detector performance over long periods of routine use.

Image Credit: ELTRA
Applications include coke production, analysis of graphite materials, coal quality control, and environmental monitoring. The system has been specifically designed for the consistent analysis of conditions and routine laboratory operation.
Iron Ore Mining: Loss on Ignition Using Thermostep TGA

Image Credit: ELTRA
Loss on ignition is commonly used to assess moisture, hydrated minerals, carbonates, and other volatile components in iron ore analysis. These components have the potential to influence processing behavior.
LOI determination is generally performed by heating samples to 1000 °C and holding this temperature for approximately one hour. These conditions result in the release of volatile constituents while iron oxides remain stable.
ISO 11536 describes this procedure and is commonly used as a reference method.

Image Credit: ELTRA
The Thermostep TGA applies this principle in an automated thermogravimetric system. Samples are heated according to defined temperature programs, under controlled atmospheres, with continuous mass recording.
Automated cooling supports routine laboratory operation and reduces handling steps.
The system can simultaneously process up to 19 samples, plus a reference crucible. The furnace operates at temperatures up to 1000 °C and allows the use of oxygen, nitrogen, or air as process gases.
The Thermostep TGA is also used in coal testing for automated proximate analysis in line with ASTM D7582.
ELTRA Instruments in Mining Laboratory Operation
Analytical instruments in mining laboratories are often integrated into routine workflows and operated continuously. ELTRA systems are widely used in this environment, seeing daily use in laboratories to support production control, exploration, and quality assurance.
Instrument design is focused on stable operation under a range of challenging conditions, including elevated sulfur contents, abrasive samples, and variable matrices.
It is also necessary to design furnace systems, gas paths, and detector components for sustained use with limited interruptions for maintenance.
Automation options help maintain consistent analysis conditions across different shifts and operators while supporting the handling of large sample numbers.
These capabilities see ELTRA instruments being widely used in laboratories working with extended or 24-hour operating schedules.
ELTRA supports standardized analytical procedures across various mining applications by offering elemental analysis and loss on ignition within a coordinated product range.
The company’s portfolio of instruments is widely recognized in the industry and integrated into laboratory infrastructures at mining operations across the globe.
Ready to start your next project?
Get expert advice from the team
Acknowledgments
Produced from materials originally authored by ELTRA.

This information has been sourced, reviewed, and adapted from materials provided by ELTRA.
For more information on this source, please visit ELTRA.