The DVS Vacuum is an advanced gravimetric system that is designed to support both dynamic and static experiments.
It provides dual sorption solutions, carrying out dynamic experiments for lower sorbate pressures including the Henry region as well as static experiments for higher sorbate pressures.
Figure 1 shows the principles of dynamic vacuum, where controlling the entry and exit flows of sorbate helps in realizing dynamic vacuum conditions.
Advantages of Vacuum Dynamic Gravimetric Vapor Sorption
When performing experiments in the Henry region, which utilizes low sorbate pressures, operating in the dynamic mode provides two key benefits; sorbate compositional certainty, and residence time control. Figure 2 shows the sorbate molecules and substrate under dynamic and static conditions.
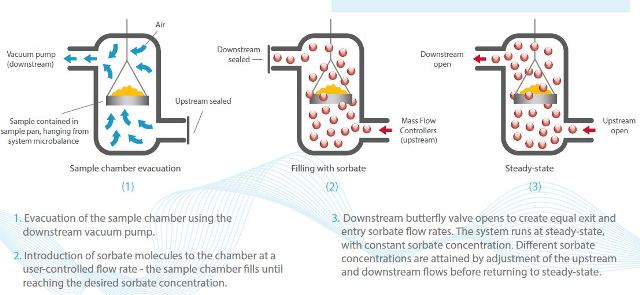
Figure 1. The principles of dynamic vacuum
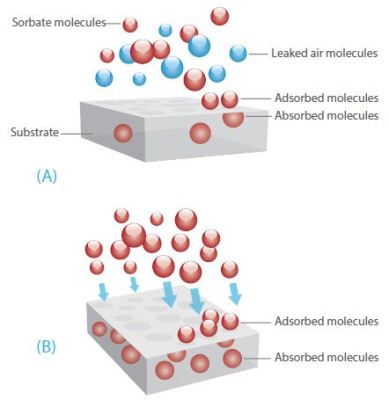
Figure 2. Sorbate molecules and substrate under static conditions (A) and dynamic conditions (B). The measured sorbate pressure would be the same in each case.
Sorbate Compositional Certainty
Static solutions that use low sorbate pressures may have small air leaks in the sample chamber which tend to dilute the sorbate molecules over time. The chamber’s overall pressure becomes the sum of contributions from the leaked air and solvent. As a result, the actual concentration of the sorbate may be lower than specified by the system sensors and this may result in experimental inaccuracies.
When the DVS Vacuum is used in dynamic mode, it ensures non-stop elimination of leaked air and its replacement with sorbate. Therefore, sorbate molecules represent the total measured pressure present in the sample chamber and provide confidence about the experimental concentration in the Henry region.
Residence Time Control
The DVS Vacuum is capable of reaching a steady-state across a broad range of flow rates. This not only provides sufficient control over the sorbate residence time with the sample, but also allows adequate contact between the sorbate and substrate to precisely determine sorption kinetics and pore size distributions.
Key Features
The main features of the DVS Vacuum are as follows:
- Multi-component/competitive sorption experiments: Conducts multi-component experiments utilizing two gasses, one gas and one vapor, or two vapors
- High vacuum: Experimental background pressures as low as 7x10-6mbar
- High-temperature sample pre-heater (optional): Pre-heats samples up to 400°C under high vacuum
- Vapors and gasses: H2, SO2, CO2 Ar, Nitrogen, Methane, Toluene, Water, Ethanol, Octane, Cyclohexane
- Henry region (low sorbate concentration) experiments: Deliverable sorbate pressures down to 0.1mbar (lower pressures available by consultation with SMS)
Selected Applications Data:
Competitive Sorption and Zeolite Studies
DVS Vacuum multi-component experiments can reproduce the industrial isolation of compounds by means of Zeolite substrates. Using the optional sample pre-heater of the DVS Vacuum, the Zeolite is activated Examples comprise the removal of SO2 gas from water vapor and the purification of organic solvents by their vapor-phase separation from water (Figure 3 and 4).
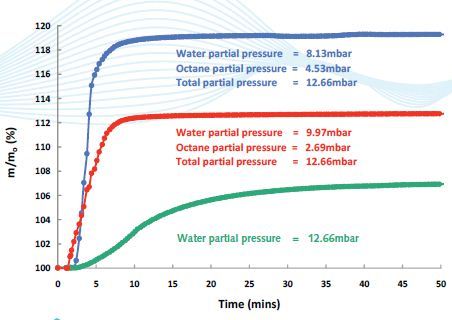
Figure 3. Octane/water competitive sorption on activated carbon coated with Chitosan: The substrate shows a strong preference for sorption with Octane, making it a potentially useful agent for the separation of organics from water.
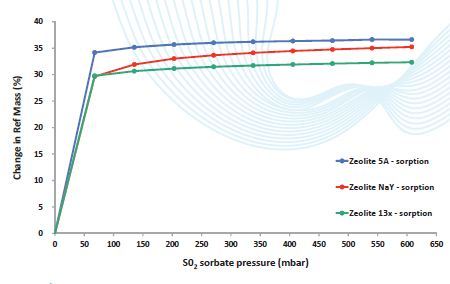
Figure 4. S02 gas sorption on three different Zeolites shows typical Type I isotherms. Sorption was found to be irreversible at 25°C – desorption.
BET Measurements at Ambient Temperatures
DVS Vacuum provides several advantages when compared to standard volumetric BET analyses, including the capability to utilize adsorbates with a variety of molecular shapes, size, cross sections, plus the capability to carry out measurements at ambient temperature conditions rather than at -196°C.
This makes it possible for scientists to investigate the subtle physicochemical characteristics of complex microporous materials. A typical example of BET measurements is shown in Figure 5.
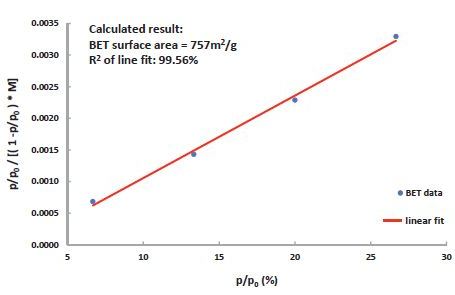
Figure 5. BET surface area of a 13x Zeolite measured by DVS Vacuum using S02 gas. The result correlates well with the established value of 750m2/g, measured using Nitrogen at –196°C.
Metal Organic Framework Studies
Metal organic frameworks (MOFs) are crystalline clusters of metal ions coupled by organic linkers. They have large specific surface areas and nanometer-sized pores which enable high-capacity storage of compounds and natural gasses.
As a result, MOFs are used in a number of critical environmental applications such as the capture of CO2 emissions and fuel storage for natural-gas powered vehicles, nano-reactors, catalysis, and drug delivery. Figure 6 shows the CO2 sorption on a MOF substrate.
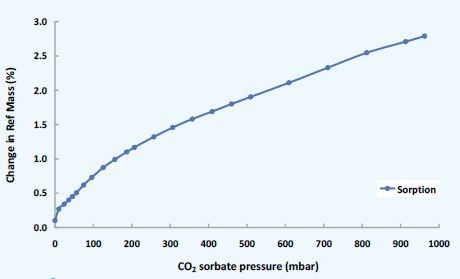
Figure 6. CO2 sorption on MOF substrate: Isotherm measured at 25°C.
The low experimental pressures in the DVS Vacuum make it possible to activate these nano-porous materials. Moreover, the system can reproduce 'real-life' MOF applications by using CO2, H2 and CH4.
Measurement of Vapor Pressure and Heat of Sublimation of Solids Using a Knudsen Cell
This is a robust and powerful technique and is described in the Organization for Economic Cooperation and Development (OECD) Guidelines for the determination of vapor pressure (OECD Guideline 104). The Knudsen Cell (Figure 7) is a crucible that has an orifice of known dimensions.
The sample is held in the Knudsen Cell and following exposure to high vacuum, the material exits via the hole by way of a sublimation process and results in mass loss (the vacuum eliminates back scattering by air molecules).
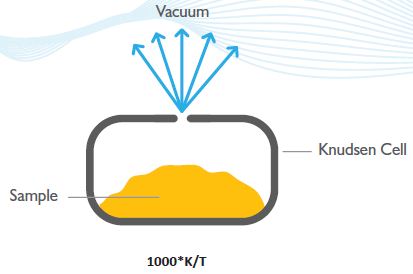
Figure 7. The Knudsen Cell
Using the measurement of the rate of mass loss as well as other experimental factors, the vapor pressure in the sample can be determined by applying the Knudsen equation. The measurement of the vapor pressures of solids is applicable in a number of applications.
Examples comprise fragrance research into air fresheners, storage of toxic chemicals, and pharmaceutical research on drug stability. Figures 8 and 9 show the vapor pressure of Bifenthrin and the vapor pressure of caffeine, respectively.
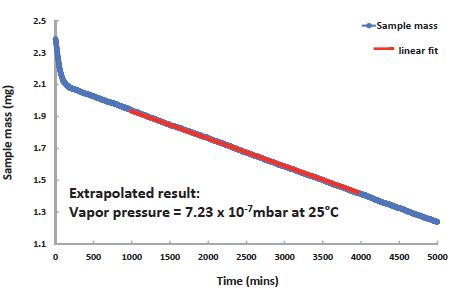
Figure 8. Vapor pressure of Bifenthrin is too low to be measured at 25°C, but can be extrapolated from the DVS Vacuum data recorded at 65°C;
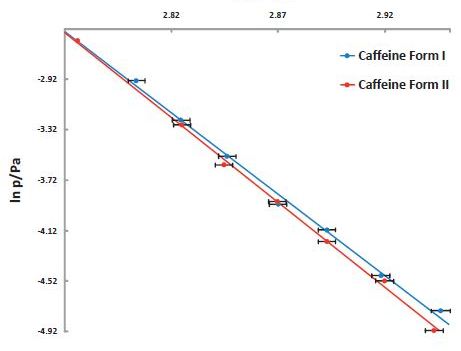
Figure 9. Vapor pressure of Caffeine vs temperature stability study. The lower vapor pressure of Form II shows it to be the more stable polymorph.
Specifications
Temperature-controlled incubator sets the experimental temperature between the ranges of 20-70°C. A high-temperature sample pre- heater, which is optional, pre-heats the sample up to 400°C under high vacuum.
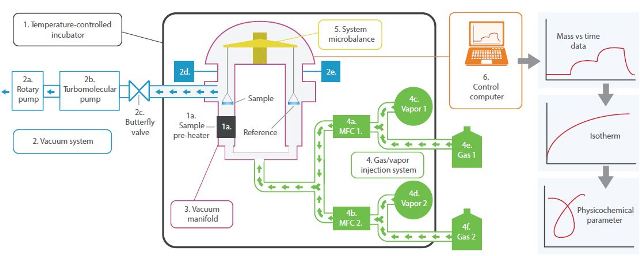
Figure 10. Schematic diagram of the DVS Vacuum.
The vacuum system includes a rotary pump and a turbomolecular pump. The former creates a vacuum down to 1.3x10-3 mbar, while the latter provides an additional vacuum down to 7x10-6 mbar in tandem with a rotary pump.
The vacuum system also includes a butterfly valve that controls the exit rate of the gas/vapor sorbate from the system and offers a point of pressure control downstream from the sample chamber. Gas/vapor pressure transducers determine and feed back gas/vapor sorbate pressure, ranging from 1013mbar to 0.1mbar. Vacuum manifold is made of 316 stainless steel to ensure chemical inertness.
As mentioned before, the DVS Vacuum can utilize one vapor or gas, two vapors or two gasses, or combination of one vapor and one gas to deliver a range of concentrations for gas, water, and organic vapors. The sorbate exit rate is controlled by the downstream butterfly valve (2c) and the sorbate entry rate is controlled by the thermal conductivity mass flow controllers (MFCs - 4a and 4b).
System microbalance records the sample mass in real time as desorption and sorption of the sorbate occurs.
- Resolution: 0.1µg
- Sample mass: up to 1.0g
- Mass change: up to ±150mg
A control PC and software regulates the experimental parameters and constantly records and saves information for analysis at a later date.
DVS Vacuum Control Software:
This software makes the experimental process simple and easy. Its windows-based interface is intuitive and easy-to-use, enabling users to perform a set of different DVS Vacuum experiments from the control computer.
Key features include:
- Purge gas flow control or Vapor 2 flow control
- Vapor I flow control
- Turbo pump control
- Butterfly valve control
- Real-time display of experiment progress
- Balance tare and calibration wizards
- Each method stage may be fixed in time or determined by a user-defined dm/dt
DVS Vacuum Data Analysis Software
This software leverages the experimental versatility of the DVS Vacuum. It provides user-friendly and extensive data analysis and generates reports simply at the click of a button.
Key features include:
- Simple and easy importing of results from different techniques for concurrent analysis
- Flexible data-range selection
- Superposition of multiple data graphs
- dm/dt and Knudsen vapor pressure analysis
- Isotherm generation
DVS Analysis Suites
The DVS analysis range comes in Standard, Advanced, and Isotherm versions.
- Isotherms
- BET surface area
- Heat of sorption analysis
- Diffusion analysis
- Pore size distributions
- Activation energy analysis