Jun 29 2016
An international group of scientists from Moscow Institute of Physics and Technology (MIPT), Rostov-on-Don, China, France, and Germany have explained the movement of charge-carrying particles in perovskite. The Science journal has observed that perovskites could be used in solar batteries in the years to come. The findings of the research have been presented in the Physics Review B. These results would help scientists to discover a desired perovskite structure by taking into consideration the basic features, rather than choosing one at random.
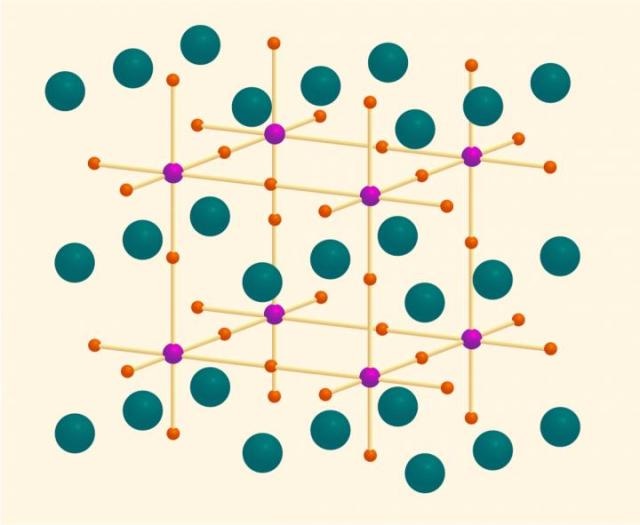 Praseodymium atoms are shown in green, oxygen atoms in orange, and titanium atoms in purple. (Credit: MIPT)
Praseodymium atoms are shown in green, oxygen atoms in orange, and titanium atoms in purple. (Credit: MIPT)
Perovskite has a near perfect structure. Due to their non-ideal structures most high-temperature superconductors use perovskite. It can also be used in the production of adaptable solar batteries without rare-earth metals. The resulting reduced costs would make large scale production of solar batteries possible.
One of the authors notes the manganite-like properties of perovskites. “This material exhibits many interesting and intriguing properties, most notably giant magnetoresistance. Many manganite properties are unknown, despite the fact that manganites have been studied for decades. We tried to work out what the conduction mechanism is of one of the most common compounds - Pr1-xCaxMnO3,” he says.
As all of these features have been discovered through experiments, the methods to explain these new features have not been discovered.
It has been over 150 years since the inital discovery of semiconductors. Electricity was also a novel concept at that time. However there were conductors like gold and copper, isolators such as glass and rubber, and other unclassified materials, semiconductors, that did not belong to any of these categories. Transistors were first made in the 1930s, when this problem was finally solved. In the modern world, an eletronic device without a transistor is hard to imagine.
However, it is not possible to track the movement of charge molecules through a microscope. The researchers from Terahertz Spectroscopy Laboratory used indirect means of detection. In order to determine the particles that are conductive, they used voltages of varied frequencies and analyzed the relationship between the induced currents and the frequencies.
They calculated the temperature dependence of permittivity and conductivity, and the frequency in a broad range of 5-3000 cm-1 in order to cover all bases. Broad temperature ranges, between 10 to 300 K or -263 to 27 °C, of samples were taken to discover similar dependences of samples with varied conduction mechanisms. However, even this measure was inadequate to evaluate the characteristics of the charge carriers. Due to this, the researchers compared perovskite with varied ratios of praseodymium (Pr) and calcium (Ca).
The team of scientists led by Boris Gorshunov, Terahertz Spectroscopy Laboratory supervisor, (Lenar Kadyrov PhD, and laboratory scientists Elena Zhukova and Vladimir Anzin are also authors of this article) realized that the charge-carriers present in Pr1-xCaxMnO3 perovskites are polarons. Polarons are electrons that move through the atoms of a material. Moving a polaron makes the nearby positive charges to move closer to it, while it makes the negative charges move away.
The features of perovskite is suitable for electron-phonon (the vibrations in a crystal lattice are called phonon) coupling, which are evaluated by the interplay of symmetry breaking communications. The team of researchers have discovered that the movements of polarons, which move as one unit, are systematic. This shows that the charge-carriers act like uncoupled particles. The concept of coherence is adopted in superconductors, quantum calculations, lasers, extremely accurate distance measurements, among others.
Determining the way that conduction takes place may help the research of perovskites and the progress of large-scale applications. One application is the perovskite-based system that is used to separate water into hydrogen and oxygen, with high efficiency. Substituting LEDs with perovskites is another potential application, however they can currently only function at the temperature of liquid nitrogen.