Jul 18 2016
If a barrier is constructed that is relatively stronger than the material attempting to penetrate it, then the barrier should be able to work - this concept seems to be fairly basic physics.
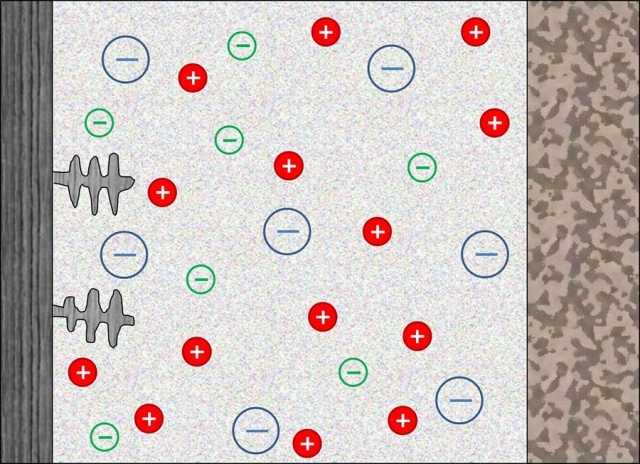 An illustration of the electrolyte inside a rechargeable battery, with the large blue spheres representing the immobile anions tethered to a solid matrix. The small red and green spheres represent the positive and negative ions, respectively; at left are dendrites, whose growth is limited by the immobilized anions. (Credit: Lynden Archer)
An illustration of the electrolyte inside a rechargeable battery, with the large blue spheres representing the immobile anions tethered to a solid matrix. The small red and green spheres represent the positive and negative ions, respectively; at left are dendrites, whose growth is limited by the immobilized anions. (Credit: Lynden Archer)
That is the current understanding behind the concept of high-capacity rechargeable batteries that employ reactive metals as the negative electrode. The membrane that is capable of separating the two electrodes should preferably be made of something that is as sturdy as the metal itself.
Because of the relatively high modulus [strength] of lithium metal at room temperature, it’s been thought for some time that the only membranes that can enable stable battery operation are ceramics. Unfortunately, few ceramics are conductive enough to meet other requirements of rechargeable batteries – including fast transport of ions, reasonable cost and manufacturability. The status quo understanding has created a technological roadblock in our ability to engineer better batteries.
Lynden Archer, Professor of Engineering, Cornell University
Conversely, Archer and a group of Cornell researchers, including CBE professor Donald L. Koch and doctoral student Mukul Tikekar, are setting new trends toward such kind of batteries. The paper titled, “Stabilizing Electrodeposition in Elastic Solid Electrolytes Containing Immobilized Anions,” has been featured in the Science Advances journal.
The study has recommended a new method, which confronts the prevailing notion that if negatively charged ions or anions are tethered in an elastic membrane of any strength, the battery could be stabilized through non-mechanical ways. In electrochemical cells that employ reactive metals as electrodes, tethered anions render stability by decreasing the electric field at the metal electrode and thus improving the stability at the time of recharge.
The latest study is based on the earlier work that focused on resolving the issues about the safety and design of high-energy batteries, particularly those based on lithium and other similar metals. Such growths are likely to experience failure because of the tree-like growths known as dendrites. Dendrites can promote short circuits, which can reduce the life of the battery and lead to fire and explosion. Increasing the strength of the membrane that separates the two electrodes is one way to arrest the chain of events which promote a dendrite-induced short. This happens to be the basis of existing practice.
A more fundamental approach would be to understand and thereby stop the nucleation event that produces the dendrites in the first place.
Lynden Archer, Professor of Engineering, Cornell University
“And the lack of viable ways to reach the present targets of stability and conductivity also underscores the need for solutions outside the box,” Tikekar added.
The latest study reveals that positive ions in the battery electrolyte spread freely across the separator whenever a battery is run at low power output. However, at higher currents, negative ions in the same battery electrolyte move away from the region close to the metal electrode, producing a depletion zone. This zone triggers the formation of dendrites. Nevertheless, the depletion zone can be neutralized by permanently binding the anions to the membrane. This will prevent the sequence of events that eventually lead to battery failure. Based on Tikekar’s calculations, this prediction has been experimentally validated beforehand and the strategies used for stabilizing metal deposition, demonstrated by his analysis, has considerably added to the toolset available to materials researchers.
“While tethering anions can slow the development of dendrites, it is the combination of elastic stresses with fixed anions that leads to the complete suppression of dendrites,” Koch said.
The researchers are particularly excited about the fact that besides lithium, this approach can also be used on other materials, such as aluminum and sodium.
It turns out that pretty much any battery that uses an energetic anode has this problem [of dendrite growth], and so the fact that in a single framework we can capture the physics that are in part responsible for it and propose methods for addressing it, it’s kind of a big deal. We hope to deepen our understanding by devising experiments to test the model more rigorously.
Mukul Tikekar, Doctoral Student, Cornell University
The study reported in the Science Advances journal was supported as part of the Energy Materials Center at Cornell, an Energy Frontier Research Center funded by the U.S. Department of
Energy.