Aug 23 2016
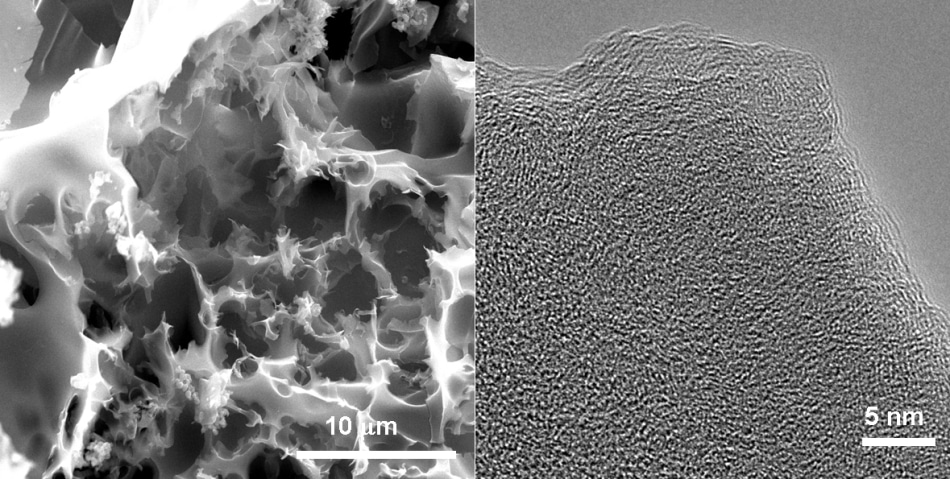 High-resolution transmission microscope (left) and scanning electron microscope images of a porous carbon sample studied for its ability to capture carbon dioxide from natural gas. (Credit: Barron Research Group/Rice University)
High-resolution transmission microscope (left) and scanning electron microscope images of a porous carbon sample studied for its ability to capture carbon dioxide from natural gas. (Credit: Barron Research Group/Rice University)
A team of researchers from Rice University have found that a calculated balance of various components in carbon-capture materials would help to isolate greenhouse gases and simplify the processing (sweetening) of natural gases.
The project was led by Andrew Barron’s lab, and it aimed to map how carbon capture is affected by changes in porous carbon materials and the atmosphere in which they are synthesized. They discovered aspects that could help to improve the products of the industry, and minimize costs.
The findings of the research will be presented in the August issue of the Royal Society of Chemistry’s Journal of Materials Chemistry A.
The scientists studied how the characteristics of porous carbon, manufactured in the form of pellets, influence carbon dioxide capture. Barron said that the results are influenced by various factors including pressure, temperature, size of the pores, the elements added and the surface area of the material. He added that the map developed will impact further research in the area of carbon capture.
The traditional sense has been the more surface area and the greater the porosity of the material, the better it will adsorb. So people have been synthesizing materials to maximize both. It turns out that’s kind of a dead area of research because once you get to a critical number, no matter how high you get after that, they don’t improve absorption. What we’ve done is provide a recipe to make carbon capture materials the best they can be.
Andrew Barron, Chemist, Rice University
A wide variety of porous carbon materials were created by the scientists using sawdust and pulverized coconut shells that were treated with potassium hydroxide to create nanoscale pores in the grains. A few batches, enhanced with sulfur, and nitrogen, were explored as additives that increase the absorption of materials.
A wide range of precursors were used to synthesize porous carbon-based sorbent materials that are chemically activated at 500°C to 800°C or 932°F to 1472°F. The scientists then measured the carbon capturing capacity of these materials at pressures ranging between 0 and 30 bar, where one bar is just less than the average atmospheric pressure at sea level.
The experiments demonstrated that irrespective of the functional additives, the maximum surface area and pore volume achieved are 2,800 m2/g and 1.35 cm3/g, respectively. After reaching this level, an increase of surface area or pore volume did not result in increase in the efficiency in carbon capture.
“Trying to make something with a higher pore volume doesn’t help,” Barron said. “Higher surface area doesn’t help. Once you get to a certain point, no matter what you do, you’re not going to get any better with a certain material.”
The scientists also found that the conditions ideal for trade-off between methane and carbon selectivity are not ideal for carbon capture. Barron stated that the perfect material should capture all the carbon dioxide and allow all the energy-loaded methane to pass through.
The barrier where it doesn’t help you any more is different for the total uptake of carbon dioxide than it is for the selectivity between carbon dioxide and methane. Industry doesn’t have to be making the highest-surface-area material. They just have to make it with a surface area that reaches maximum production.
Andrew Barron, Chemist, Rice University
The scientists found that a material that is enhanced by oxygen, instead of sulfur or nitrogen, and has less than 90% carbon, was best suited for both methane selectivity and carbon capture; specifically materials that are activated at temperatures nearing 800°C.
Materials that had a surface area of more 2,800 m2/g were the most efficient in capturing carbon at 30 bar pressures. However, the efficiency gained with high surface area deteriorated when the pressures were less.
The researchers also discovered that oxygen, which is introduced by the pore-inducing potassium hydroxide, rather than sulfur or nitrogen was more relevant to the outcomes.
We understand oxygen is important. We don’t understand why. Does it stabilize certain pore structures? Is it because it stabilizes the pore neck? Is it changing the shape of pores? We don’t know whether it’s a chemical or physical issue, but now we know what we should study next.
Andrew Barron, Chemist, Rice University
Saunab Ghosh, a postdoctoral researcher from the Rice University is the lead author of the research paper. Co-authors of the paper include researcher Marta Sevilla and Professor Antonio Fuertes of the Spanish National Research Council, Oviedo, Spain; Senior Lecturer Enrico Andreoli of the Energy Safety Research Institute, Swansea University, Wales; and Jason Ho, an engineer at the Apache Corp., Houston. Barron is the Charles W. Duncan Jr.–Welch Professor of Chemistry and a professor of materials science and nanoengineering at Rice and the Sêr Cymru Chair of Low Carbon Energy and Environment at Swansea.
The study was supported by the Welsh Government Sêr Cymru Program, the Ministry of Economy and Competitiveness of Spain, the European Regional Development Fund, Apache and the Robert A. Welch Foundation.