Particle size specifications are usually required for suspensions or solid dosage forms, because particle size affects many aspects of product performance. To this end, setting realistic and meaningful specifications is an important aspect of Quality by Design (QbD). Specifications that are clearly defined control the performance of products because they derive from associations between clinical behavior and the variables quantified routinely during processing and for quality control. This article evaluates the process of setting specifications, taking particle size as an example which is a vital parameter for a wide range of pharmaceutical formulations.
The pharmaceutical industry encounters multiple pressures and the regulatory bodies that control this sector are encouraging the change towards a risk-based approach of production. QbD constitutes a major part of this shift in emphasis, promoting knowledge development through which producers will prove their capability.
Setting Effective Product Specifications
According to the FDA guidance specified in ICH topic Q6A, specifications are important quality standards that are proposed and justified by manufacturers and endorsed by regulatory authorities as conditions of approval. Since specifications define the acceptability of a product, the requirement for one is based on the existence of a correlation between a variable and some aspect of performance. Within the structure of QbD, such links establish a parameter as a Critical Quality Attribute (CQA), the control of which ensures the quality of product. These may include:
- Particle size
- Polymorphic form/amorphous content
- Physicochemical properties such as refractive index, pH, melting point
- Water content
- Microbial content
- Chiral identity
- Inorganic impurity levels
Evidently, when a variable has no impact on product quality, it is not necessary to specify values for it, but where there is a correlation any related specification must provide sufficient control.
Particle Size Specification
A decision tree in the ICH topic Q6A ascertains the necessity for a particle size specification, suggesting them for solid dosage forms as well as for liquids comprising undissolved drugs. Particle size can be important for any of the following properties:
- Dissolution
- Processability
- Stability
- Product content uniformity
It is a well-known fact that dissolution behavior depends on the size of a particle, or more precisely on surface area. Finer powders dissolve more quickly, and powders with a narrow size distribution dissolve at a similar rate. For orally inhaled and nasal drug products (OINDPs), the correlation with bioavailability is more direct, because particles that are wrong in size will not deposit within the preferred part of the respiratory system, and fail to enter the body by the prescribed route.
Processability is very critical, as it is linked to the consistency and quality of products, with poorly controlled processes creating a low quality output. Particle size relates to segregation behavior, powder flowability, and compressibility. Hence, size specifications that are clearly defined for intermediates or in-process material may provide a way of reducing process variability.
Stability issues usually occur because the forces acting on particles are a virtue of their size. Smaller particles for instance are more likely to agglomerate because inter-particle forces are comparatively high. In the case of suspensions, the tendency to settle increases with size due to the effects of gravitational forces. Stability is an important parameter to consider during the development of particle size measurement techniques.
Finally, the size of the particle can affect blend uniformity, and consequently the dose content uniformity related to a product. For instance, Figure 1 shows a NIR chemical image of a recalled tablet with the active ingredient-rich domains highlighted in red. The right side of the tablet has larger areas when compared to the left, indicating that composition is not consistent.
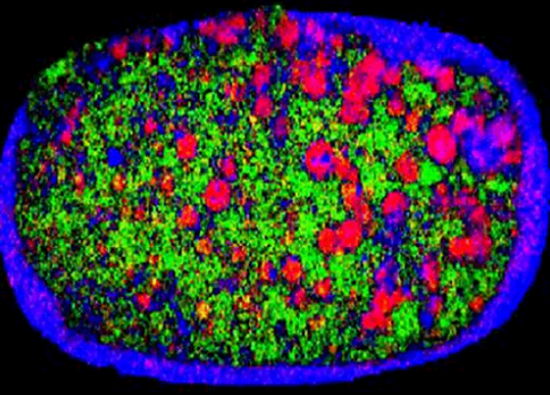
Figure 1. NIR image of a tablet showing heterogeneous distribution of the active ingredient.
To sum up, particle size specifications are usually required for suspensions or solid dosage forms, as particle size affects many aspects of product performance.
Measurement of Particle Size Distributions using Laser Diffraction
A variety of particle size analysis tools are available to support pharmaceutical development. One of the most popular methods used for particle size analysis is laser diffraction. This method has broad applicability and is ideal for both wet and dry systems, with particle size ranging from 0.1 to 3500µm. An attractive feature of this technique is that it is a suitable Process Analytical Technology, so specifications devised in the laboratory can be transferred into production.
Particle Size Distribution
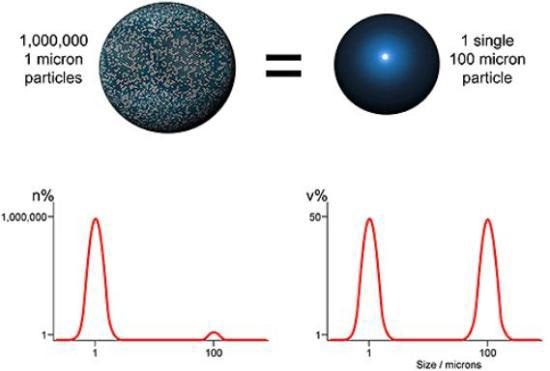
Figure 2. Comparing volume and number based distribution.
Laser diffraction is an ensemble sizing method and creates volume based distributions. This means that data is produced concurrently for the entire sample (ensemble), rather than for separate particles, and is represented with respect to the volume of material in a given size fraction. An alternative, utilized by microscopy and imaging techniques for instance, is to show data on a number basis, that is, display the number of particles in each size fraction. Both techniques are equally suitable, but the results obtained are very different (Figure 2).
Selecting aa Appropriate Parameter
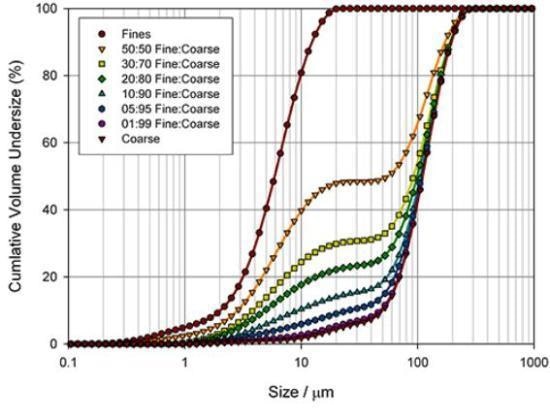
Figure 3. Assessing the ability of a laser diffraction analyzer (Mastersizer 2000) to identify coarse and fine particles in a pharmaceutical blend.
One way to determine the best basis for a specification is to measure the sensitivity of different particle size parameters to changes in a sample. Figure 3 illustrates particle size distributions, quantified for the blending of two excipient grades, produced by means of a Mastersizer 2000 laser diffraction analyzer from Malvern Panalytical.
Setting Appropriate Tolerances
Laser diffraction provides excellent robustness, repeatability and reproducibility, creating good quality data with little manual input. Setting suitable tolerances for a specification needs an appreciation of measurement errors, together with an understanding of the association between the variable and product performance.
Figure 4 illustrates some particle size distribution data, the orange and yellow lines measuring reproducibility and the red line being a typical reading. If the specification for this product is a Dv50 of 10 µm, then the plot demonstrates that the measurement variability will be +/- 5%. However, it would be incorrect to assume that the same tolerance can also be utilized for the percentage below 10 µm.
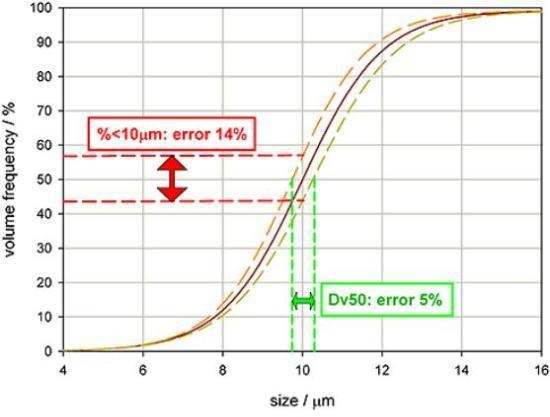
Figure 4. Highlighting how accuracy is affected by the basis or wording of a specification.
Owing to the slope of the undersize distribution curve, a specification demanding that 50% of the volume of material must be 10µm in size or less would be subject to +/-14% measurement variability. This analysis clearly underlines a point that as measurement variability increases, assurance in the reported result should reduce.
Conclusion
Setting meaningful and realistic specifications on the basis of product understanding is a major aspect of QbD and important for successful quality control. To this end, particle size in many pharmaceuticals is a Critical Quality Attribute that must be strongly controlled, as it directly affects performance.
Laser diffraction is ideal for a variety of applications within the pharmaceutical industry and is sensitive to over-sized materials. This technique is also well-established as a process analytical method.
Mastersizer laser diffraction analyzer offers highly repeatable results (+/-1%) and reduces the need to tighten tolerances to account for measurement errors.

This information has been sourced, reviewed and adapted from materials provided by Malvern Panalytical.
For more information on this source, please visit Malvern Panalytical.