Formulation development is an important activity for a wide range of industries, from paints and coatings to pharmaceuticals. In the UK alone, it has been estimated that sales of formulated products come to roughly £180 billion per annum 1. Therefore, with significant value attached to formulation, it is very important to get it ‘right.’
Stability is an important attribute for many products, directly impacting on kerb appeal, performance, shelf-life, and ultimately, worth. Therefore, a systematic approach to understanding and developing stability can help to improve the chance of success in long-term stability trials and ultimately speed up formulation.
Malvern Panalytical’s Zetasizer range is considered the world’s most extensively used system for colloid, nanoparticle, and protein size and zeta potential measurements. This article discusses how these capabilities can be leveraged to develop stable dispersions.
What Do We Mean by Stability and Why is it Important?
When it comes to emulsions and suspensions, stability refers to the tendency of the dispersed phase to remain unchanged and evenly distributed across the continuous liquid phase over time. Coagulation or aggregation, sedimentation or settling, and the joining together of one of more particles are key mechanisms of instability.
These mechanisms may take place either independently or in combination, just like the case of aggregation followed by associated sedimentation of the resulting larger particles - a common problem.
Optimizing properties to meet a specific application is the defining goal of formulation. If a resulting formulation is unstable, these optimized characteristics are easily lost or eroded. For instance, in the case of a suspension medicine, a homogeneous particle distribution ensures uniform dosing, while the size of the dispersed drug particles is linked to in vivo dissolution and bioavailability.
Both clinical efficacy and safety may be compromised if the drug particles aggregate and/or sediment. However, in an ink or paint, the size of the dispersed particles influences the way in which light interacts with the finished coating – observed tint or hue, transparency and/or level of gloss – and practical features such as ease of application and weather resistance. In such cases, aggregation has a direct impact on performance, consistency, and value.
A critical aspect of formulation is stability testing. It defines the period of time over which a customer can expect the product to provide consistent, optimized and/or safe performance, setting a specification for shelf life. To maximize the safety, practicality, and commercial appeal of the product, understanding and controlling stability is important to extending a product’s shelf life as far as possible.
The following sections describe five strategies that can be adopted with the Zetasizer Nano to realize that goal.
Characterize Particle Size under Relevant Conditions
Measuring particle size at or near to neat sample concentrations detects the onset of aggregation under relevant conditions.
The size of particles in suspension is not essentially the size of the particles used to make up the suspension. Determining the size of particles in the undiluted formulation helps to detect any tendency towards aggregation under ‘in-use’ conditions.
Making Measurements
Using the dynamic light scattering (DLS) technique, the Zetasizer Nano measures particle size across the range 0.3 nm to 10 µm. Small particles in a suspension/dispersion are subject to Brownian motion. The rate of Brownian motion can be directly determined from the scattered light pattern created by the moving particles.
DLS systems determine the particles’ rate of diffusion from measurements of fluctuations in the intensity of light scattering and change the result to particle size/particle size distribution data using the Stokes Einstein equation.
DLS is highly sensitive to the presence of low levels of aggregates or larger particles because light scattering intensity correlates with particle size to the power six.
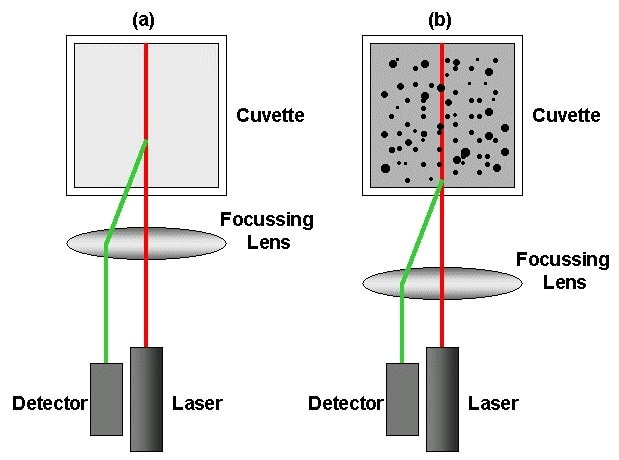
Figure 1. Schematic showing the key components of a DLS system – laser, detector, focusing lens and cuvette (which holds the sample) – in a NIBS configuration.
Instrument Features that Add Value
Non-invasive backscattering (NIBS) technology – This detects scattered light at a large angle relative to the incident beam (173° in the case of the Zetasizer Nano) which not only increases sensitivity but also extends the range of concentration over which accurate size data can be collected by reducing multiple scattering.
Multiple scattering is when the scattered light interacts with more than one particle before detection. Within the Zetasizer Nano, NIBS technology also includes high-quality movable optics that automatically optimises the focus position of the lens to reduce multiple scattering, improve signal to noise and thereby increase accuracy for all samples. The overall result is accurate, relevant particle size data across a concentration range running from 0.1 ppm to 40% w/v, enabling measurement of the widest range of formulations without any need for dilution.
Example Data: Measuring Emulsion Droplet Size as a Function of Sample Concentration
Droplet size measurements of a pharmaceutical emulsion were made across a range of sample concentrations, from 0.001 to > 5 %w/v, to evaluate the effect of concentration on recorded values (Figure 2). The results demonstrate that the measured droplet size data is independent of sample concentration, proving the instrument’s ability to deliver consistent data as the sample concentration, and the possibility for back scattering, increases.
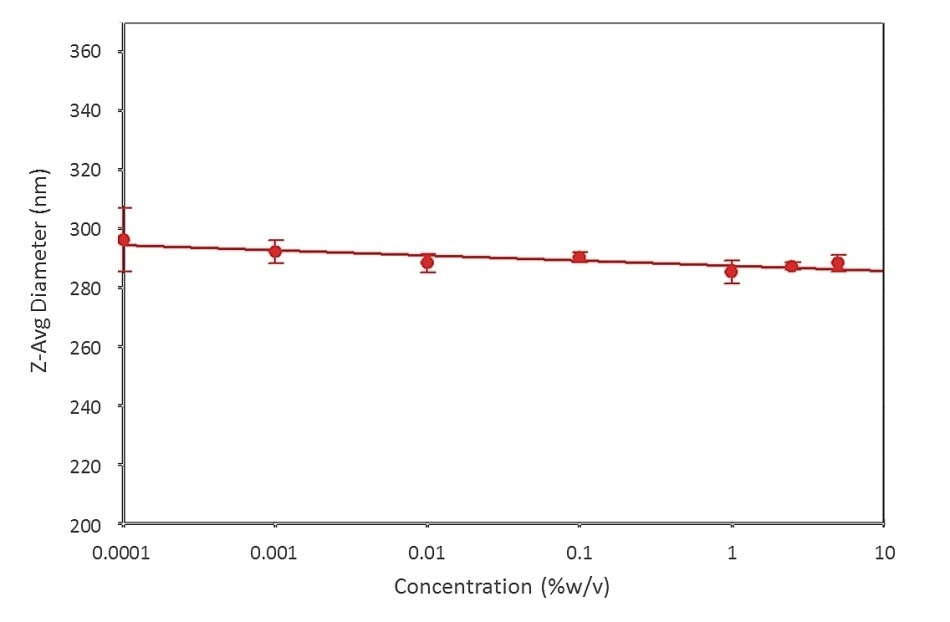
Figure 2. Data for a pharmaceutical emulsion show the ability of the Zetasizer Nano to reliably and repeatably measure particle size over a wide range of sample concentrations, using NIBS technology.
Measure Zeta Potential
Zeta potential is directly related to electrostatic stability, providing the information needed to optimize the effect of charge within a dispersion.
Zeta potential is defined as a measure of the magnitude of electrostatic forces of attraction or repulsion between particles in a dispersion. When particles repel, the risk of instability and aggregation is reduced. A zeta potential of high magnitude, preferably greater than +/- 30 mV, is linked to greater stability. A zeta potential of low magnitude, relatively close to 0, indicates that particles tend to attract, and consequently aggregate.
Making Measurements
Laser Doppler micro-electrophoresis or electrophoretic light scattering (ELS) technique is used to measure zeta potential. When an electric field is applied to a suspension/dispersion, any charged particles present move in a direction and at a velocity that is related to their zeta potential. Velocity measurements help to calculate electrophoretic mobility, and from this, zeta potential.
Instrument Features that Add Value
A configuration for both DLS and ELS measurements – the Zetasizer Nano has been designed to measure both zeta potential and particle size, increasing its value for stability assessment.
M3-PALS technology – for precise determination of zeta potential, the speed of particle movement should be measured accurately. A patented laser interferometric technique known as M3-PALS (Mixed Mode Measurement - Phase Analysis Light Scattering) is used by the Zetasizer Nano to deliver unprecedented sensitivity 2. M3-PALS helps to analyze samples with low electrophoretic mobility and their mobility distributions are precisely and free of electro-osmosis effects.
Example data: Using Zeta Potential Measurements to Optimize Silicone Emulsion Stability
Silicones show a high degree of thermal stability, and are consequently integrated in an array of medical and household products in the form of emulsions. Zeta potential measurements of three different silicone emulsion samples were compared with observed stability rankings to establish whether zeta potential can be applied to predict the stability of a fourth sample.
The results display a close correlation between the measured zeta potential and observed stability with just one sample (#3) showing a zeta potential indicative of robust electrostatic stability (Figure 3). Based on the results, the stability of the fourth sample, which exhibited a measured zeta potential of 13.2 mV, can be predicted to have poor stability, closely analogous to that of sample A.
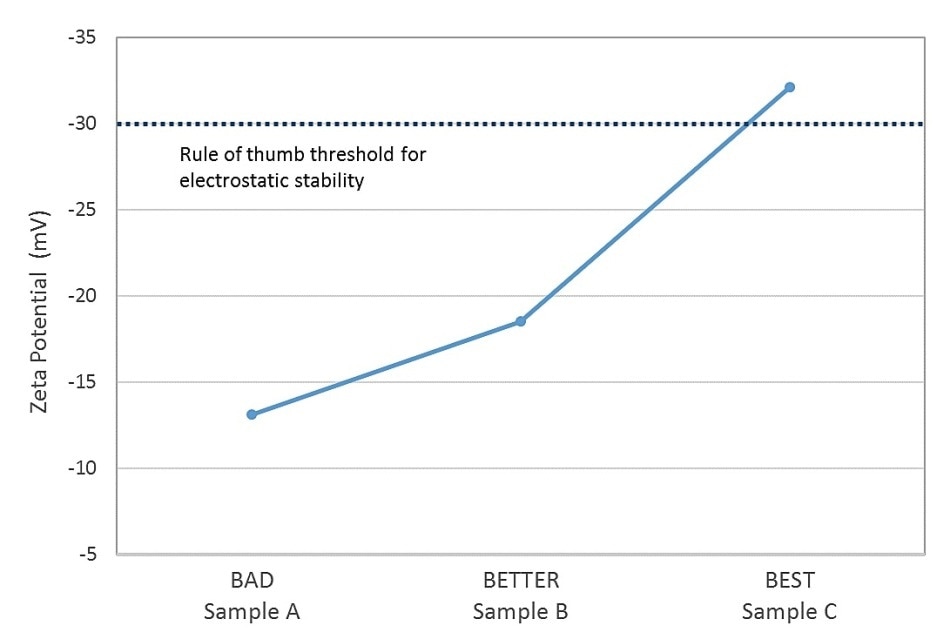
Figure 3. Data for silicone emulsion samples show a close correlation between observed stability and zeta potential values.
Measure Stability Prediction Parameters
Measuring the DLS interaction parameter, kD, alongside zeta potential supports the robust prediction of long term suspension stability.
The DLS interaction parameter (kD) is a measure of the propensity of a larger molecule to isolate reversibly into smaller components – the opposite of aggregation. It is affected by both hydrodynamic and thermodynamic interactions between particles in a suspension.
A more positive kD is indicative of a strong repulsive interactions, and consequently, greater stability. Measuring kD under relevant conditions provides an understanding of formulation stability provided by zeta potential.
Making Measurements
kD is measured from the concentration dependence of the diffusion coefficient of the sample, as established from the DLS data using the following expression:
Dm = D0 (1+kdC)
where Dm represents the mutual (measured) diffusion coefficient, D0 is the self-diffusion coefficient (the diffusion coefficient at zero concentration), and C represents the concentration of the sample. So, it can be easily determined through a series of DLS measurements.
Instrument Features that Add Value
Advanced software – the Zetasizer Nano has software that calculates KD from test measurements, providing easy access to the required data.
Example data: Assessing the stability of HSA dispersions as a function of pH
Dispersions of Human Serum Albumin (HSA) are used routinely as a biological reference standard, and are deployed in a wide range of medical applications, for instance to restore blood volume. The effect of pH on the stability of HSA dispersions was established by generating kD values at a pH of 4 and 7, via dynamic Debye plots of the diffusion coefficient as a function of concentration (Figure 4). Based on the resulting data, if the acidity of dispersion is increased, it leads to better stability.
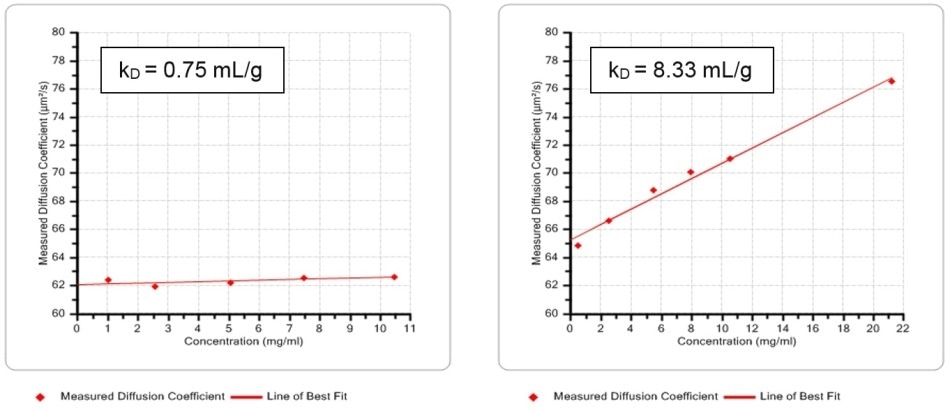
Figure 4. Dynamic Debye plots for HSA at pH 7 (left) and pH 4 (right) with lines of best fit for determining kD . The results show that the dispersion is more stable under more acidic conditions.
Explore the Influence of pH, Ionic Strength and Component Concentration
Efficiently scoping the influence on stability of pH, ionic strength and composition accelerates progress to a formulation that meets stability targets.
A better understanding of how different components can affect stability is required to manipulate the composition of a formulation to meet stability targets. An effective alternative to a trial and error approach is to measure zeta potential and/or particle size as a function of the parameter of interest.
Making Measurements
The impact of pH, ionic strength, and other similar parameters requires repeat measurements of samples that differ with respect to the parameter of interest. Automation can help to considerably reduce the manual input needed for such testing, while improving instrumental productivity.
Instrument Features that Add Value
An autotitrator - such as the MPT-2 titrator accessory for the Zetasizer Nano, carries out automatic conductivity, pH, and additive titrations, which accelerates stability investigations. The MPT-2 titrator accessory automatically changes sample composition before transferring it to the optics unit for measuring particle size and zeta potential. It also allows automated measurement of the IEP (isoelectric point) – the point of zero zeta potential.
Example data: Assessing the stability of Intralipid mixtures as a function of calcium chloride concentration
Intralipid is a triglyceride oil-in-water emulsion that is used for parenteral nutrition either alone or combined with other components, such as minerals and vitamins, to form a total parenteral nutrition (TPN) solution. A plot of the zeta potential of Intralipid emulsions as a function of calcium chloride is shown in Figure 5.
Calcium chloride was used to replicate the effect of addition of a mineral. The results demonstrate that in the absence of calcium chloride, the Intralipid shows high stability – a zeta potential value of about -50 mV. However, when small quantities of calcium chloride are added, this stability is rapidly eroded, with an IEP observed at about 4 mM concentration.
Further additions of calcium chloride result in a charge reversal in zeta potential indicating that the Ca2+ ions attach to the surface of the emulsion droplets.
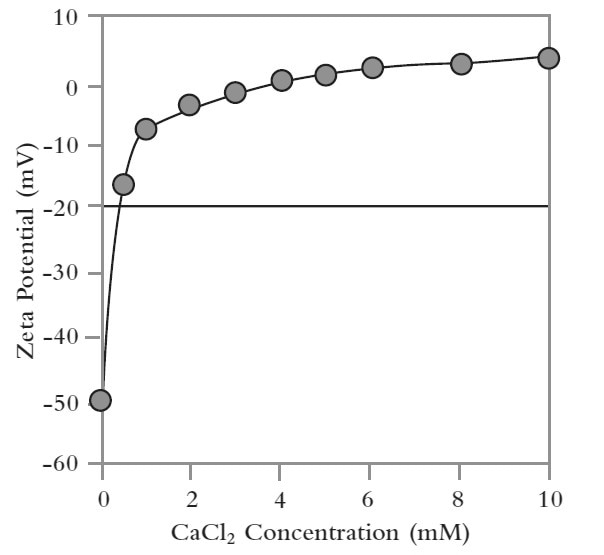
Figure 5. A plot of zeta potential as a function of calcium chloride concentration shows how the addition of the salt compromises stability.
Determine the Impact of Temperature
Temperature can directly impact formulation stability, making it essential to scope any effect.
Extremely high temperatures can change the stability of formulation by, for instance, reducing the continuous phase viscosity so that sedimentation may take place, or accelerating the movement of particles that may possibly increase aggregation.
Therefore, investigating the effect of temperature is imperative for formulations that are meant for use or storage at high temperatures. However, on a wider level, the stability of closely similar formulations can be differentiated by testing at an elevated temperature.
Making Measurements
Similar to assessing the effect of composition, the effect of temperature can be explored by making repeated measurements under closely controlled conditions with automation being useful in easing the analytical burden.
Instrument Features that Add Value
Built in, high performance temperature control – This is an efficient alternative to an external water bath and circulation system and provides better results. For all DLS measurements, good temperature control is required because the viscosity of the dispersant and the Brownian motion of a particle directly depend on temperature.
The Zetasizer Nano provides integral Peltier temperature control over the range of 0 to 90 °C to +/-0.1 °C (0 to 120 °C for the High Temperature model), in addition to rapid system warm-up and the ability to quickly change temperature – both features that can be helpful for studies at elevated temperatures.
Example data: Assessing the impact of temperature on paint stability
Stability is quantified by measuring the zeta potentials of two paint samples (#305 and #803). Different chemical polymerization methods were used to synthesize the samples and, in use, #803 was observed to be stable, whereas #305 was not, with a deposit forming in the paint after several months.
For both samples, the zeta potential values were 55.0 and 51.1 mV respectively, and neither of these results or the size data determined at ambient temperature gave any indication as to why the paints may perform differently. At 80 °C, repeat measurements of size were performed to see whether higher temperature testing would differentiate them (Figure 6).
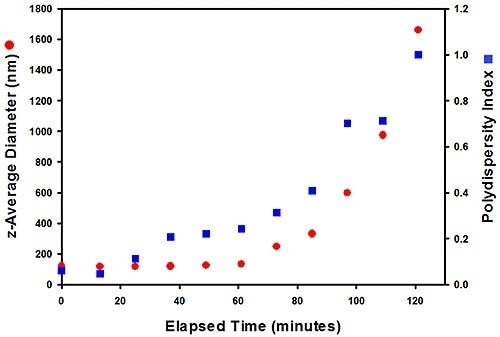
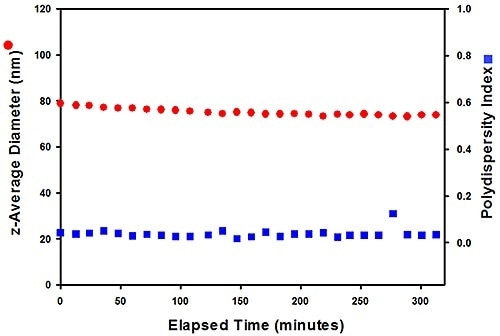
Figure 6. Size data for the #305 sample (top), measured at 80 °C, show that it aggregates over time, while #803 (right) has greater stability.
The results were measured at 80 °C which revealed that after 40 minutes, sample #305 starts to aggregate, while in sample #803 the size of particles remains constant over four hours, showing its superior stability. Here, the correct testing helps to detect a difference in stability over a relatively short timescale, relative to the months of storage needed to observe an effect in use.
Introducing Zeta Potential: A Reliable Indicator of Dispersion Stability
Zeta potential measurements are often used to evaluate the stability of a wide range of colloidal systems. Zeta potential is defined as a measure of the magnitude of charge or electrostatic repulsion at the boundary layer surrounding a particle, and is one of the primary parameters known to affect the stability of a system.
Particles within a solution are surrounded by two layers of ions – the outer diffuse layer and the tightly bound inner Stern layer – where ions are less firmly associated. There is a notional boundary within the diffuse layer known as the slipping plane beyond which the particle exerts minimal influence. Charge at the slipping plane is called the zeta potential.
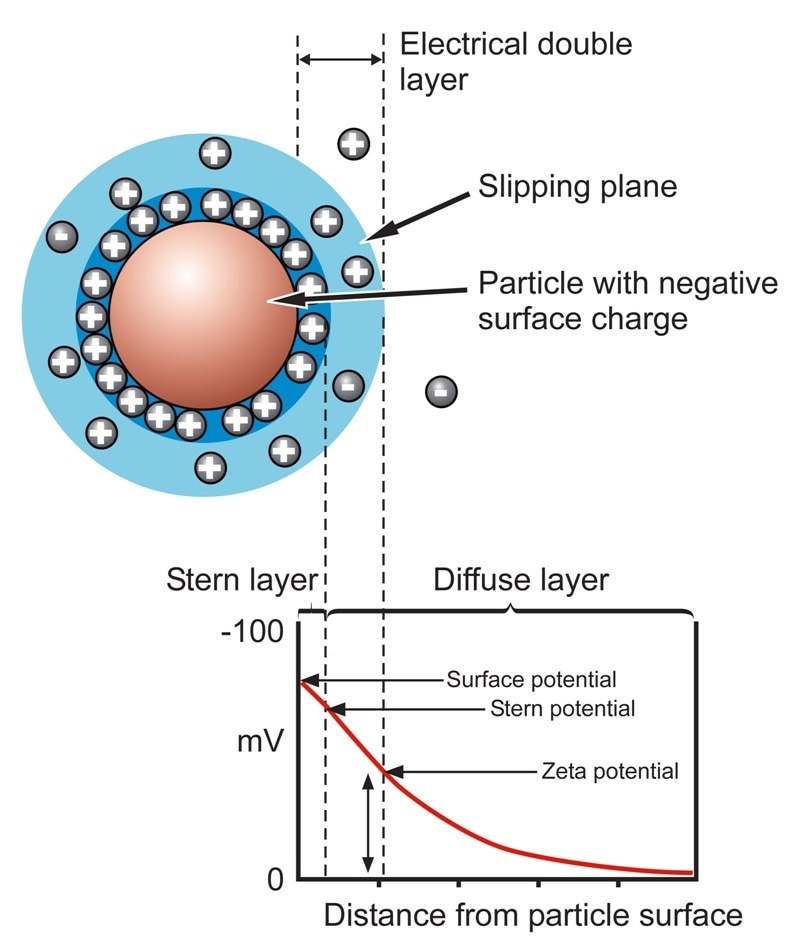
As a result, zeta potential measures the balance of attractive and repulsive forces that are experienced by the particles as they approach one another. At a zeta potential close to zero, these forces are low, the system is not stable and is likely to aggregate. On the other hand, a marked positive or negative zeta potential (+/-30 mV) indicates an electrostatically stable system that resists particle aggregation.
References
1. https://connect.innovateuk.org/web/formulation1
2. Simplifying the measurement of zeta potential using M3-PALS - Available for viewing at the Malvern Panalytical website
Time to find out more
The Zetasizer Nano can benefit formulators by providing multiple measurement protocols, which instead of trial and error approach support an efficient knowledge-led one and also rapid progress towards an optimized formulation that fulfills performance targets. Please read on in the following areas to learn more about the value of Zetasizer Nano for your application:
Zeta potential: An introduction in 30 minutes – Available for viewing at the Malvern Panalytical website: http://www.malvern.com/en/support/resource-center/technical-notes/TN101104ZetaPotentialIntroduction.aspx

This information has been sourced, reviewed and adapted from materials provided by Malvern Panalytical.
For more information on this source, please visit Malvern Panalytical.