Malvern Panalytical’s recent peer-reviewed publication, Tailoring Lipid Nanoparticle Dimensions Through Manufacturing Processes, published in RSC Pharmaceutics, features both the NanoSight Pro alongside Zetasizer Advance Ultra, demonstrating the strength of these complementary technologies in the characterization of lipid nanoparticles (LNPs).
Image Credit: Kateryna Kon/Shutterstock.com
This collaboration showcases how combining nanoparticle tracking analysis with dynamic light scattering can provide deeper insights into LNP formulation and manufacturing processes.
Lipid Nanoparticles
Lipid nanoparticles are at the forefront of drug delivery, particularly in the creation of mRNA vaccines and other sophisticated therapies.
The capability to precisely characterize these nanoparticles is critical for improving their design, formulation, and production methods. This is where the collaboration between NanoSight Pro and Zetasizer Advance Ultra truly shines.
Power of Two
The paper highlights the importance of understanding physicochemical attributes for optimizing LNP-based drug formulations and evaluating their effects in vitro and in vivo.
Key parameters such as particle size, polydispersity, and zeta potential are crucial for assessing formulations. These attributes offer insights into formulation stability (via zeta potential) and heterogeneity (via polydispersity index or span).
When identifying trends, a multi-technology approach is essential to validate findings. For example, NanoSight Pro is an ideal complement to Zetasizer Advance, as it delivers rapid particle size distributions. In addition, NanoSight Pro’s visual validation offers immediate and direct confirmation of sample heterogeneity and potential stability issues.

Image Credit: Malvern Panalytical

Image Credit: Malvern Panalytical
While both techniques measure size distribution, nanoparticle tracking analysis (NTA) often reports smaller particle sizes than dynamic light scattering (DLS), particularly for larger, more polydisperse LNP samples (see figure below).
DLS, being intensity-based, is influenced by larger particles dominating the overall distribution, whereas NTA’s particle-by-particle approach avoids this bias. For smaller, more uniform LNPs, size distribution data from both methods align well.
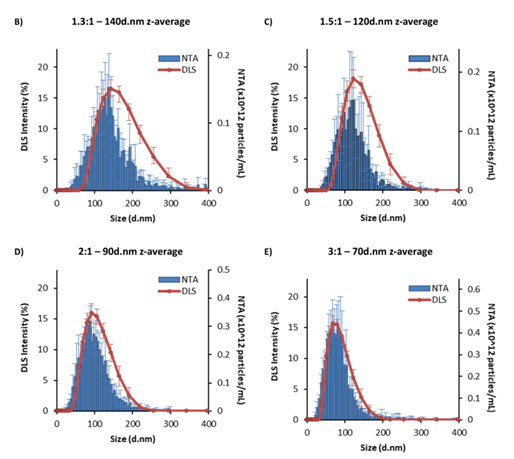
Fig 2. NTA vs. DLS data. (A) Number of particles per mL in 1 mg mL-1 LNP formulation. (B-E) NTA concentration vs. DLS intensity plots at four different aqueous: organic phase ratios. NTA data (blue) and DLS traces (orange). The z-average is the intensity mean given by the DLS for each sample. The LNPs were manufactured with an N/P ratio of 6 from 5 mg mL-1 lipid stock and purified by dialysis in pH 7.4 PBS for 1 hour. Each measurement is a mean of three independent batches with error bars showing +SD. The significance is indicated by an asterisk (*) between each of the phase ratios analyzed by one-way ANOVA with Tukey's pairwise comparisons. NTA = nanoparticle tracking analysis; DLS = dynamic light scattering. Image Credit: Caitlin McMillan, Amy Druschitz, Stephen Rumbelow, Ankita Borah, Burcu Binici, Zahra Rattray and Yvonne Perrie, RSC Pharm., 2024, 1, 841-853
NTA also provides concentration measurements, allowing researchers to determine whether LNPs formed as expected. This study found that smaller LNPs had greater particle counts than bigger ones, indicating a consistent material distribution across samples.
The study focuses on particle size as a key quality attribute (CQA). Size influences not only the manufacturing process but also the expression efficiency of LNP-based therapeutic candidates, both in vitro and in vivo.
Complementary Insights
By combining the strengths of NanoSight Pro and Zetasizer Advance, researchers can achieve comprehensive characterization of lipid nanoparticles. This approach supports more informed decision-making across the entire development pipeline—from vector design and formulation optimization to large-scale manufacturing.
The implications are significant. Improved characterization tools can accelerate the development of safer, more effective medicines, leading to faster delivery of life-saving treatments and better health outcomes for patients.
Sharing Research
“I am proud to see how NanoSight Pro and Zetasizer Advance play a pivotal role in this cutting-edge research. A lot of care goes into developing features that solve problems and help our customers overcome their challenges. However, once the instruments leave our hands, we don’t often get to see them in action, particularly in the pharmaceutical industry, where confidentiality is paramount due to the high stakes involved. This is why it is especially rewarding to read research like this, which highlights the real-world impact of our technologies.” Product Manager for NanoSight Pro
Acknowledgments
Produced from materials originally authored by Agnieszka Siupa at Malvern Panalytical.

This information has been sourced, reviewed and adapted from materials provided by Malvern Panalytical.
For more information on this source, please visit Malvern Panalytical.