
Alongside the US, many countries are accomplishing improved clean air standards. Reliable sulfur detection and sampling are key to meeting the required levels. This is how SilcoTek® is improving sulfur detection and supporting industry in meeting clean air standards across the globe.
Consequences of Air Pollution for Health and the Environment

A number of years ago, I visited friends in Pittsburgh. Although we explored many fantastic points around the city, it was a single black and white photo taken at the inclined plane on Mount Washington that struck me the most. The image, judging by the cars, was taken around 1940 and was of a lively downtown Pittsburgh at what seemed to be early evening.
The scene was gray, dim and murky, and cars were using their headlights. I was shocked by the caption: Downtown Pittsburgh at 12 Noon! During that time, the air quality was so poor that in order to avoid accidents, cars were forced to use their headlights at midday. Never mind blue skies, you were fortunate to see where you were going!
This reminded me of a story my mother told me about growing up in Pittsburgh. If she left the window open while sleeping, by morning, the sill would be blackened with soot and there would be grime under her nose. She told me that simply to keep up with all the inky particulates from the mills close by, my Grandmother had to have the wallpaper cleaned annually.
Being raised in such polluted conditions is something I cannot imagine. Sadly, a significant number of the planet’s population is all too familiar with such scenes, but change is taking place and the benefits of clean air are being recognized globally.
To illustrate, China has established vigorous new standards to improve air quality, recognizing the advantages of increased standards of health, a more powerful economy and improvements to the functioning of society. They understand that decreases in air quality related deaths, flight disruptions, and plant shut downs related to pollution are beneficial for both China's economy and quality of life in general.
Indeed, the idea that clean air is important seems to be spreading globally, as demonstrated by the number of new, stricter air standards being established across the world:
- Tier 3 (US) (related methods include ASTM Method D5453, ISO Method 20846, and ASTM Method D2622)
- National 5 and Beijing 6 (China)
- Bharat Stage 6 (India)
- Euro 5 and Euro 6 (EU)
- Clean fuel projects (Middle East)
- IMO Global Sulfur cap (Global shipping, effective 2020)
These recent air quality standards share a leading aim: the reduction of the impact of pollution through the detection and control of particulates, low level sulfur (SOx), and NOx emissions.
How NOx and Sulfur Compounds Add to Air Pollution
Recall the photo of Pittsburgh at noon? Well, the cause of all that gloom was smog. Sulfur dioxide (SO2), and to a lesser extent SO3, as well as oxides of nitrogen (NOx), formed the gray haze. However, the dark cloudy appearance is not the only concern. For both the environment and humans, SOx and NOx exposure have a true and enduring effect, including:
- Lung disease and breathing difficulties
- Depression of immune system
- Defoliation caused by acid rain
- Damage to structures from acid exposure
If that sounds awful, how about this statistic? In 2015, nine million deaths globally resulted from pollution, due largely to smog. Poor air quality causes almost one third of deaths in China. With this information, it is unsurprising that air pollution control has become a large-scale initiative across the developed world.
The key to management of SOx and NOx is reliable detection. It would be impossible to measure the performance of air pollution controls like scrubbers and clean fuels without a way to sample and compare pollution levels. Unfortunately, sulfur is an element which is difficult to detect, as it has a tendency to adhere to common materials used in sample flow paths, including all sorts of metals, glass, and ceramics.
Why Is It Challenging to Sample Sulfur?
Sampling and detection are difficult because sulfur, and sulfur compounds, often adhere to materials commonly used in sample flow paths. The instrument cannot reliably record the data if the sulfur gets trapped in the flow path, making control of scrubbers and processes problematic.
For an illustration of how sulfur can get lost in this way, Shell and O'Brien administered a study in which a sulfur compound was injected into a tube. They then waited for the detector at the opposite side to "see" the sulfur. They waited and waited, and at last, after 90 minutes, some sulfur hit the detector! This is a graph of the test results.
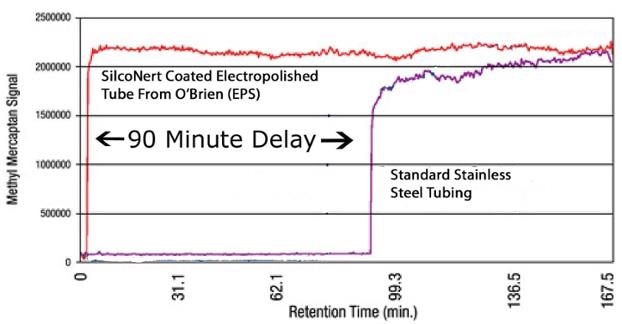
The initial red line is a graph of the sulfur response when that tube is coated with SilcoNert®. Under identical test conditions, the response is almost instant, with practically no sulfur lost in the tube. When all of the sulfur reaches the detector, the plant or refinery can accurately manage emissions or their process; making for an improved product while minimizing sulfur-related air pollution.
How significant an issue is sulfur adsorption? If we consider the US EPA Tier 3 standard, a 10ppm sample will be entirely lost in a relatively short length of sample tubing. If the sample were exposed to a high surface area metal, for example, if a sample were filtered through a sintered metal frit, much, if not the entirety, of the sample would be adsorbed onto the surface.

To demonstrate how dramatic sulfur loss can be, a sample of hydrogen sulfide, carbonyl sulfide, and methylmercaptan were injected onto a GC column through a three inch long stainless steel liner. Throughout the short exposure to the stainless steel liner, the sulfurs adsorbed onto the stainless steel surface. The adsorption was so exhaustive that the detector was not able to pick up any hydrogen sulfide (H2S) or mercaptan (image below).
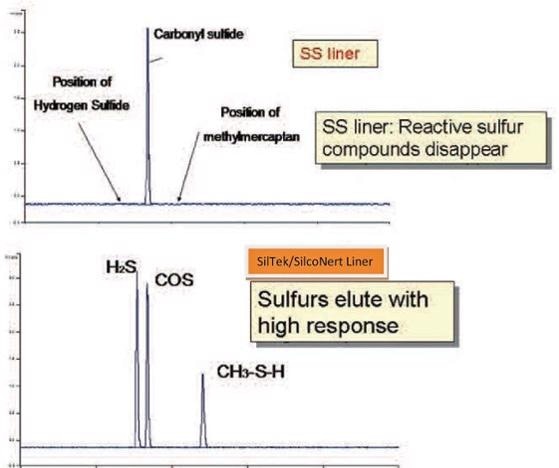
If the test is repeated using a SilcoNert® 2000 coated liner, the H2S and mercaptan are plainly seen by the detector, as shown in the second graph.
How Sulfur Adsorption Can Be Stopped in Sampling and Instrumentation Flow Paths
SilcoTek®’s inert CVD coating, SilcoNert®, improves sulfur sampling and air quality by providing a barrier between the sample and the reactive stainless steel surface. This lets the sulfur or NOx sample pass directly through the flow path, delivering the whole sample to the detector without interacting with the surface. The benefits of this inert coating include:
- Increased sensitivity
- Improved sample system reliability
- Higher levels of precision and repeatability
- Improved resistance to corrosion and moisture
In some instances, how long you can hold the sample is more important than how quickly you can deliver it. When field operations staff take a grab sulfur sample, perhaps from the well or from the refinery hydrotreater, they can experience sulfur adsorption problems. From the second the sample enters the sample cylinder, its stainless steel surface will adsorb sulfur, therefore, time is crucial when attempting to accurately assess if the well is sweet or sour, or if the hydrotreater is running to specifications.
In a stainless steel cylinder, sulfur stability can be measured in minutes or hours. Beyond this, the sample is unlikely to be indicative of field conditions. In contrast, SilcoNert® coated stainless steel sample cylinder stability is measured in days or weeks. This means that instead of hurrying a sample back to the lab, field technicians can grab and carry a sample for 30 days or more, confident that it is undifferentiated from when it was taken from the field.
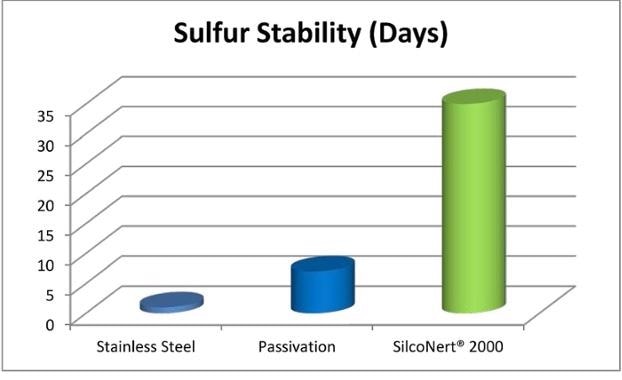
Accurate and quick sulfur response allows the plant to better control pollution emissions and avert plant upsets or issues with regulatory compliance. Better sulfur and NOx sampling flow paths provide the plant operator with the tools to reach those stricter clean air standards globally.

This information has been sourced, reviewed and adapted from materials provided by SilcoTek.
For more information on this source, please visit SilcoTek.