PTFE and other fluorinated polymers are thermally stable, chemically inert, and extremely hydrophobic because of their intrinsically low surface energy.
The chemical inertness of PTFE, for example, means that it is virtually impossible to form lasting adhesive bonds. Instead, coatings tend to form droplets and 3D particles on the surface, preventing the formation of a uniform film.
Conventional approaches for increasing surface energy concern the use of aggressive primers, which are an ecological hazard.
Fluorinated Polymers | PTFE
Fluorinated polymers are not readily modified by standard plasma processes.
Oxygen plasmas are utilized effectively for treating many hydrocarbon-based polymers but are not successful for fluorinated polymers due to the binding energy of the fluorine to carbon atom being considerably greater than that of oxygen to carbon.
Plasma treatment of fluorinated polymers using oxygen plasmas leads to a gradual etching of the surface instead of surface activation.
However, the hydrogen plasma creates an instant and lasting modification to the nature of the fluoropolymer surface. The action of atomic hydrogen, produced by the plasma, is to react with surface fluorine and extract this into the gas phase, where it is subsequently pumped away by the vacuum system.
Following this, hydrogen terminates the free surface bonds to create a CHx polymer surface that is readily wettable. The resultant surface is also mildly etched on a microscopic scale, generating a microscopically structured surface. The combination of both actions results in a changed surface that may be glued, painted, etc.
Image Credit: Henniker Plasma
Typical Results
The results presented below demonstrate the change in morphology, obtained surface energy, and change in water contact angle for PTFE after hydrogen plasma treatment.
The surface energy of PTFE following plasma treatment is lower than that of many common polyolefins, such as polypropylene and polyethylene, and well within the range of wettability needed for various adhesives to form strong bonds.
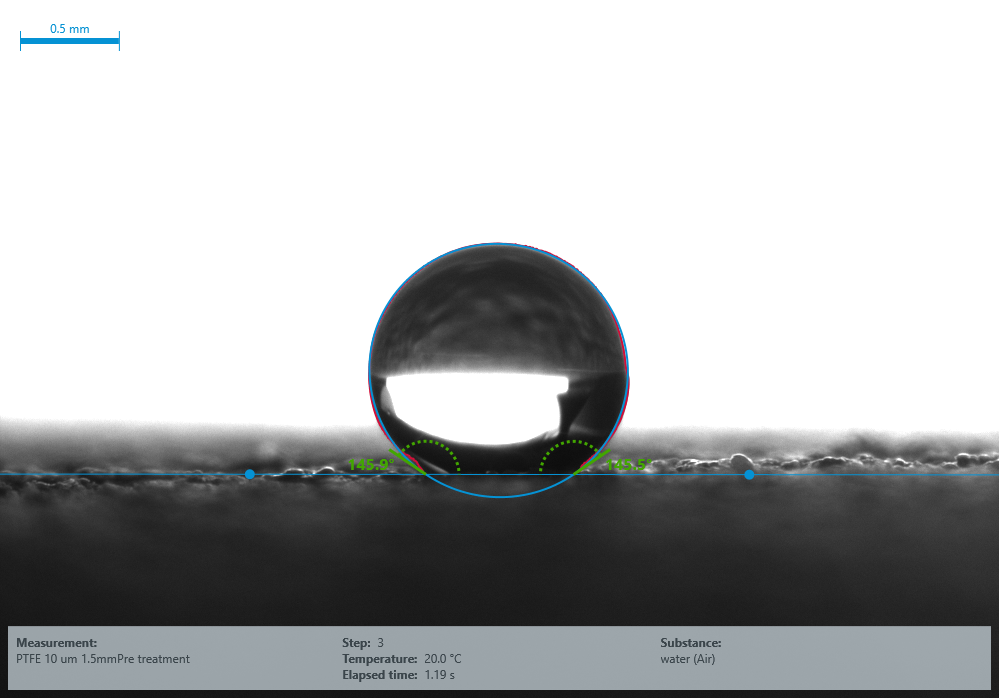
145° Water contact angle for untreated PTFE (Surface Energy 10 mN/m). Image Credit: Henniker Plasma
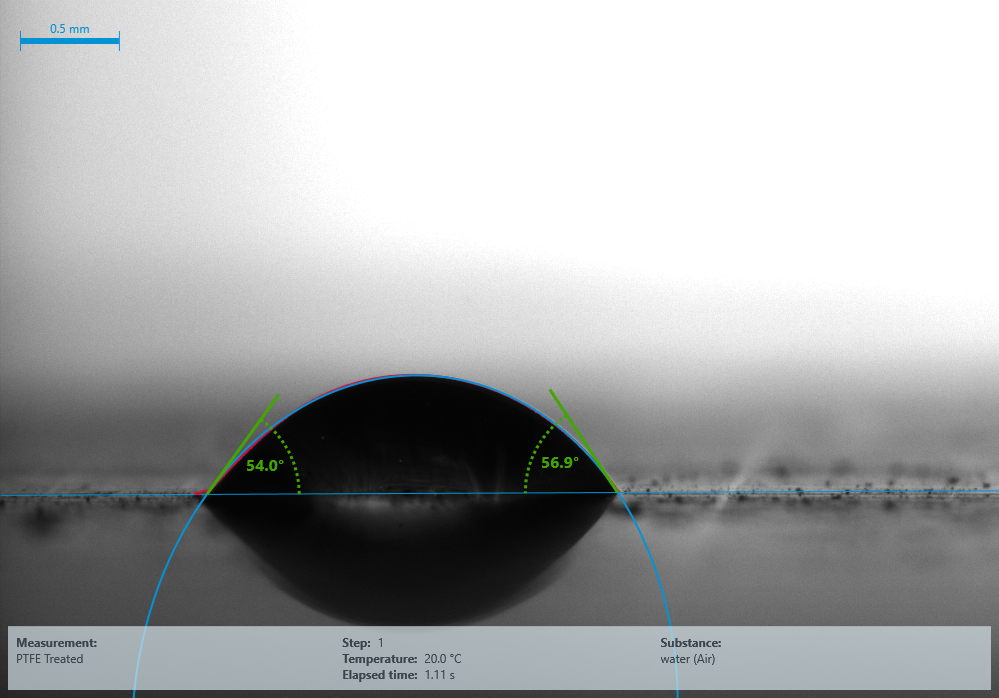
54° Water contact angle for treated PTFE (Surface Energy 51 mN/m). Image Credit: Henniker Plasma
PTFE Surface morphology before and after hydrogen plasma treatment.
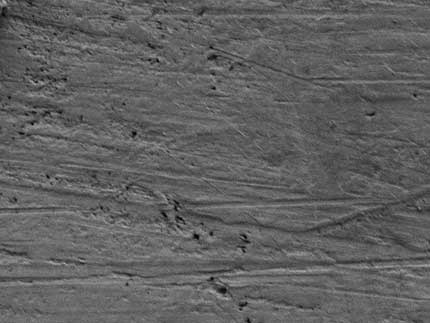
Untreated PTFE. Image Credit: Henniker Plasma
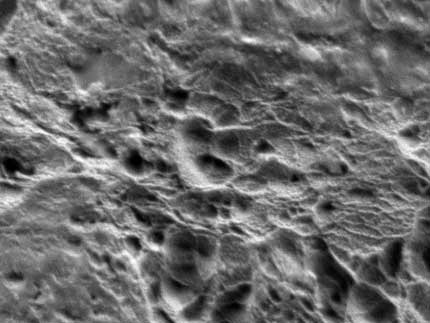
H2 Plasma Treated PTFE. Image Credit: Henniker Plasma
Visual comparison of surface wettability of PTFE before and following hydrogen plasma treatment.

Untreated PTFE. Image Credit: Henniker Plasma

H2 Plasma Treated PTFE. Image Credit: Henniker Plasma
Plasma Process
The ionization of gas atoms leads to the collision of high-energy particles knocking electrons out of their orbits. This causes the characteristic “glow” or light linked with plasma.
Plasmas have various species such as molecules, atoms, electrons, ions, free radicals, metastables, and photons in the short wave ultraviolet (vacuum UV or VUV) range.
Plasmas are produced in closed vessels at low pressures, typically < 1.0 Torr. The low pressure causes a long mean free path of the plasma species, enabling them to remain reactive until contact with a surface. The overall chamber temperature at the generally used pressures and power levels is close to room temperature.
Henniker HPT-100 Plasma Treatment System. Image Credit: Henniker Plasma
“Treatment of PTFE with low-pressure hydrogen plasma is a very effective method of rendering the surface wettable without using harsh chemicals.”

This information has been sourced, reviewed and adapted from materials provided by Henniker Plasma.
For more information on this source, please visit Henniker Plasma.