Henniker Plasma, a leading manufacturer of plasma surface treatment equipment, presents Plasma Activated Water (PAW).
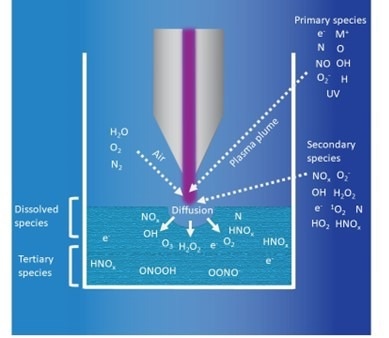 Schematic of the active species produced in PAW using an atmospheric plasma jet with process gas M. Image Credit: Henniker Plasma
Schematic of the active species produced in PAW using an atmospheric plasma jet with process gas M. Image Credit: Henniker Plasma
Plasmas operating at atmospheric pressure in ambient air produce a wide range of reactive chemical species. When an atmospheric plasma is allowed to interact with water, a unique mixture of biochemically reactive chemistries occur, and the resultant chemically active water is referred to as Plasma Activated Water (PAW). Due to its unique biochemical activity, PAW is a promising technology in food, agricultural and biomedical industries and is a possible environmentally friendly alternative to chemical fertilisers. PAW has been shown to be effective at food decontamination, seed germination and improved plant growth.
In this short contribution, we report on PAW created using Henniker’s ‘Cirrus’ atmospheric plasma system. We present measured changes in pH, conductivity, and nitrate levels as a function of plasma-water interaction time and, in collaboration with Hiden Analytical, we report membrane inlet mass spectrometry (MIMS) measurements of dissolved species in PAW using the Hiden HPR-40 dissolved species analyser (HPR-40 DSA). The effectiveness of the PAW was tested by comparing the growth and longevity of cut roses in samples of PAW and untreated water.
Plasma-Water Chemistry
Atmospheric plasma generates reactive oxygen and nitrogen species (RONS) that interact with, and are incorporated into, water. The types and relative concentration of RONS are dependent on the method of plasma generation and on the specific gas used. Typical long-lived species can include nitrites (NO2)-, nitrates (NO3- ), ozone (O3) and hydrogen peroxide (H2O2). Short-lived species, such as hydroxyl radicals (·OH), superoxide (O2-), singlet oxygen (1O2), nitric oxide (NO·) and peroxynitrite (ONOO-), exist only for short periods with half-lifetimes of the order of a second.
The concentration of reactive species existing in the water yield a solution with higher oxidation-reduction potential (ORP), lower pH and higher conductivity than non-treated water, and have significant concentrations of acidic substances such as nitric (HNO3) and nitrous acid (HNO2). The synergistic effects of RONS in PAW is responsible for its biochemical properties and its many applications.
The figure depicts the formation of RONS with a typical atmospheric plasma jet, within the plasma plume, gas region, gas-water interface, and bulk water.
Experimental
De-ionised (DI) water was exposed to the Cirrus plasma jet to produce PAW. The plasma jet had a constant power of 310 W and was situated 5 cm away from the surface of the water. The Cirrus produces a ~10 mm diameter jet of reactive plasma species using compressed air as the process gas. The standard Cirrus design and operating parameters produces oxygen and NOx radicals with only very low concentrations of ozone.
DI water of volume 200 ml was treated for times ranging from 1 – 30 minutes. Immediately after treatment, measurements of pH and conductivity were performed using pH and conductivity probes. 30 ml of PAW was then transferred into glass vials for mass spectrometry measurements with the Hiden Analytical HPR-40 DSA membrane inlet mass spectrometer (MIMS).
The inlet probe has a thin permeable membrane that facilitates the extraction of dissolved gases and vapours from an aqueous solution through the process of pervaporation. The dissolved gases permeate through the membrane and evaporate in the vacuum side of the probe before being ionised by an electron impact ion source. The resulting parent and fragment ions are then separated by mass-to-charge ratio (m/z) using a quadrupole mass filter, and subsequently detected with an electron multiplier detector. Performing m/z scans yields the relative intensities of the constituents of the dissolved gas sample.
The instrument settings used for PAW sample analysis were 70 eV electron energy and m/z survey scans from 0 – 100 amu. The untreated (background) DI water spectrum was subtracted from each treated sample spectrum to reveal the changes in its constituents.
Results
Membrane Inlet Mass Spectrometry
The background subtracted data revealed significant changes in the intensity of the m/z=30 (nitric oxide NO) and m/z=47 (nitric acid, HNO2) peaks, which were then chosen for subsequent analyses.
The evolution of m/z = 30 and m/z 47 increases for successive plasma treatment time until ~ 12 minutes when the concentration falls and plateaus at slightly lower concentrations. No other significant changes in detected species were observed.
pH and Conductivity
A significant drop in pH was found even after short treatment times. For example, untreated DI water had an initial pH of 6.96 and after 1 minute of plasma treatment, reduced to 3.85. At successively longer treatment times, the pH continues to drop but at a slower rate, settling to values close to 3.00. Conductivity on the other hand continues to increase significantly with treatment time, with a maximum of 529 µS/cm at 30 minutes of plasma treatment.
Results
Nitrate Test
The presence of nitrate and hydrogen peroxide was also measured using colour change test strips. It was found that all treatment times > 4 minutes registered a maximum colour change, indicating nitrate levels > 500 ppm. Therefore, separate experiments using shorter treatment times were performed to identify the incremental increase of nitrates in the solution.
The difference in nitrate levels for increasing plasma treatment times is shown, up to a maximum of 3 minutes, and an approximately linear increase over the treatment period is indicated.
It should be noted that there were no visible increases in H2O2 for the parameters explored here.
Effect on Cut Flowers
Freshly cut identical roses were placed in vases with equal amounts of PAW (right vase) and untreated DI water (left vase). The roses were left undisturbed and were recorded over a 7-day period.
The effects of exposure to and uptake of PAW on cut flowers is evident. The sample exposed to PAW blooms with an extended longevity and quality. Furthermore, the rose in the PAW grew a new leaf shoot during the 7-day period, which can be seen (circled) on the lower part of the stem just above the water surface.
Discussion
The interaction of atmospheric plasma with water produces a number of complex chemical reactions which change the physiochemical properties of the liquid. The pH of plasma treated water decreases with increasing treatment time, whilst conductivity increases. Since pH is a measure of the hydrogen ion concentration in solution, and conductivity is a measure of a solution’s ability for electric current flow, these simple measurements allow quick determination of the effectiveness of PAW treatment. The production of RONS and acidic substances such as nitrous, peroxynitrous and nitric acids contribute to the increase of conductivity and decrease in pH. For the range of conditions reported here, pH plateaus around 3.00 whilst conductivity continues to increase with treatment time.
Membrane inlet mass spectrometry was used to identify dissolved species. To our knowledge, this study is the first of its kind to use MIMS as a method of identifying dissolved gases in PAW. The intensity of nitric oxide (NO) and nitrous acid (HNO2), m/z = 30 and m/z = 47 respectively, increased to a plateau with plasma treatment time. The NO is likely to be formed from the ionisation of the parent HNO2 molecule in the mass spectrometer ion source however the ration of m/z(30):m/z(47) is higher than expected and further work should aim to determine whether this is due to differences in the relative permeation/enrichment rates of different RONS through the membrane sampling material.
Using simple test strips, we have shown a significant increase in nitrate levels, even at short treatment times.
The freshness and lifetime of cut flowers is an important factor for their marketability. Cut flowers often suffer from decay around the cut surface of the stem due to microorganisms and are damaged due to the inflow of air, resulting in less absorption of water and hence shorter vase life. The PAW created in this work acts as an antimicrobial agent and the increased concentrations of nitrogen containing species also act as a natural fertiliser. These are thought to be the reasons for the positive effects.
Conclusion
Using the Henniker Cirrus atmospheric plasma system, we have created plasma activated water (PAW) with different and controllable conductivities, pH levels and reactive oxygen and nitrogen species, the latter being measured with colour change test strips and by the analysis of dissolved gases via membrane inlet mass spectrometry.
Observations of the effects of the plasma activated water upon the longevity and quality of cut flowers shows that PAW can act as a fertiliser and is bactericidal.
PAW produced using the Cirrus has applications in:
- Plant nutrition
- Plant health
- Seed treatment
- As a pH controller
- As a disinfectant