There has been a surge in the use of lithium-ion batteries (LIBs) over the past two decades as they have been employed as energy storage devices in electric vehicles, notebooks, and cellular devices.
There has been a proportional increase in interest in improving the performance of LIBs in line with the increasing popularity of the batteries.
The performance of LIBs can be affected by three components: the separator, the electrolyte (salt and solvent system), and the electrodes (anode and cathode).
The electrolyte is the medium enabling the movement of lithium ions between the anode and cathode and therefore plays a pivotal role in the LIB. Often, the electrolyte is a complex mixture of lithium salt(s) and aprotic solvents.
Factors to be considered to produce a successful electrolyte include thermal stability, chemical compatibility, ionic conductivity, viscosity, solubility, and salt dissociation.1,2
Most currently commercially available LIBs have a metal oxide cathode (i.e., lithium cobalt oxide) and a carbon anode (i.e., graphite), containing an electrolyte; this is a non-aqueous solution of LiPF6 salt, dissolved in a combination of cyclic carbonates (i.e., ethylene carbonate) and linear carbonates (i.e., dimethyl carbonate, diethyl carbonate, ethyl methyl carbonate).2,3
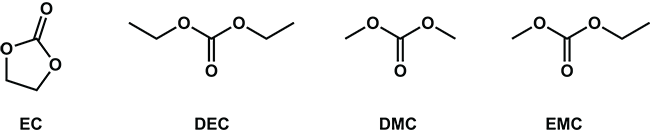
Figure 1. The general structure of the aprotic organic solvents ethylene carbonate (EC), diethyl carbonate (DEC), dimethyl carbonate (DMC), and ethyl methyl carbonate (EMC) from left to right, respectively. Image Credit: Nanalysis Corp.
It is expected that more exotic and innovative electrolyte combinations will become available as battery research progresses. A simple quantitative technique for analyzing the solvents in LIB electrolytes using benchtop nuclear magnetic resonance (NMR) is shown here.
1H quantitative NMR (qNMR) spectroscopy can conveniently be used to determine the relative amounts of each aprotic organic solvent. Using the equation below, the relative weight percentage (wt%) of each chemical species can be determined.
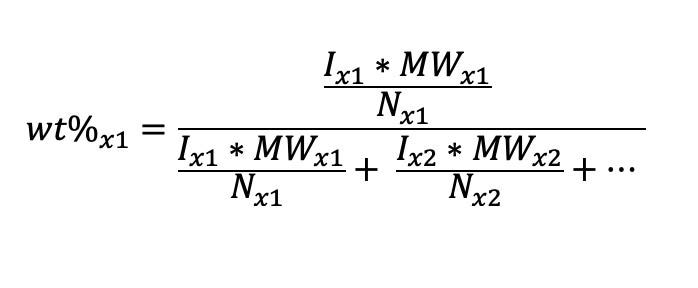
Where,
wt%x1 = the weight percentage of x1
Ix1 = the integration area of x1
MWx1 = the molecular weight of x1
Nx1 = the number of protons in x1
x1, x2, … = represents a different chemical species in solution
This is done by integrating the relative regions associated with each compound after collecting a 1D spectrum of the sample solution (without added solvent).
Figure 2 shows an example of the 1H NMR spectrum of a mixture of aprotic solvents (diethyl carbonate, dimethyl carbonate, and ethylene carbonate).
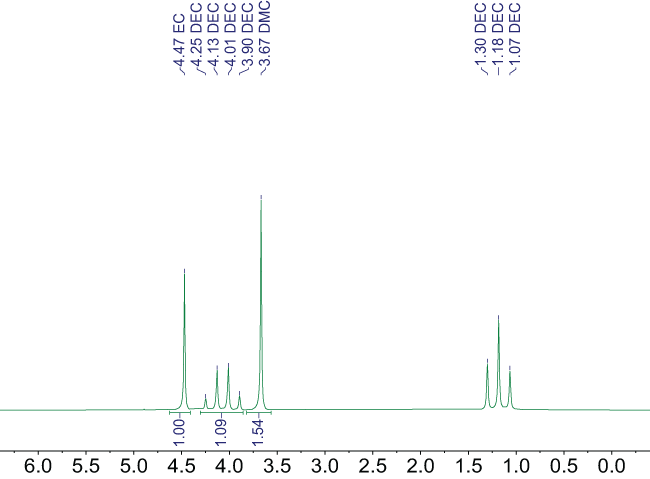
Figure 2. 1H NMR spectrum at 60 MHz of a mixture of aprotic organic solvents without deuterated solvent showing the relative integration areas of each chemical species included, along with annotations above the signals. Image Credit: Nanalysis Corp.
The following experimental parameters were used to acquire the data using the 60PRO: spectral width: 40 ppm, number of points: 16384, number of scans: 4, scan delay: 25 seconds, spectral center: 10 ppm, pulse angle: 90°, receiver gain: auto.
Precision was ensured by running each sample in triplicate.
Table 1 summarises the results on popular commercial aprotic solvents after being tested using the 60PRO benchtop spectrometer. The average value was used to determine the relative amount of each species from each spectrum.
Table 1. Comparison between the wt% obtained using the 60PRO and analytical balance. Source: Nanalysis Corp.
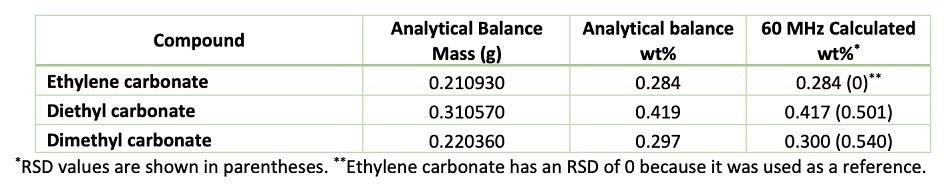
The approximate weight percentage obtained from weighing using a Mettler Toledo analytical balance (model: MS105DU) was compared to the weight determined.
Results provided by the 60PRO compare quite well to the values obtained from the analytical balance when the solvent compositions in LIBs are analyzed. Having a fast, efficient, and quantitative method at your disposal is an important asset with increasing research in energy storage.
References and Further Reading
- Mauger, A.; Julien, C.M.; Paollela, A.; Armand, M.; Zaghib, K. Mater. Sci. Eng. R Rep. 2018, 134, 1-21.
- Younesi, R.; Veith, G.M.; Johansson, P.; Edström. K; Vegge. V. Energy Environ. Sci. 2015, 8, 1905-1922.
- Nitta, N.; Wu, F. Lee, J.T.; Yushin, G. Mater. Today. 2015, 18, 252-264.

This information has been sourced, reviewed and adapted from materials provided by Nanalysis Corp.
For more information on this source, please visit Nanalysis Corp.