It is thought that without the use of fertilizers, around half of the world’s population could not be supported1. In the modern world, agriculture at a significant scale without fertilizers is no longer possible.
In order to grow enough produce for almost 8 billion people and also for industrial uses and domesticated animals, fertilizers of varying nutrient compositions are available to cater to the unique requirements of different soil types.
Information regarding the fertilizer’s composition (e.g., total nitrogen, phosphorus and potassium) is provided in order to choose the ideal fertilizer for a specific soil. Conventionally these constituents are established either gravimetrically (e.g., potassium, phosphorus, or sulfate)2–4 or with ICP-OES (e.g., phosphorus or potassium)5.
These techniques either require expensive instrumentation with high running costs (ICP-OES) or have the drawbacks of long analysis times and laborious sample preparation (gravimetry).
This article outlines how thermometric titration is an inexpensive, quick alternative technique to give information on the content of numerous nutrients in different fertilizers.
Fertilizer Composition for Different Needs
Macronutrients, the main nutrients in fertilizers, are known as phosphorus, nitrogen and potassium. Other nutrients are secondary macronutrients (e.g., sulfur) or micronutrients (e.g., boron).
Macronutrients in particular are crucial for plants, as they are required for the development of seeds and fruits, leaf growth and water transport within the plant. Most often, fertilizers are classified using their nutrient composition.
Other than single nutrient or straight fertilizers (e.g., single superphosphate or ammonium nitrate), multi-nutrient fertilizers which are made up of two or more nutrients are common, such as mono- and di-ammonium phosphate (MAP and DAP) or NPK (nitrogen-phosphate-potassium) fertilizers.

Image Credit: Metrohm AG
Aside from classification according to their nutrients, fertilizers may also be categorized into inorganic mineral fertilizers and organic fertilizers2. As their name implies, organic fertilizers are made up of organic matter which is derived from plants and/or animals, for example, dung.
On the other hand, with the exception of urea, inorganic fertilizers do not contain carbon-based materials and this article will mainly focus on inorganic fertilizers.
Conventional Determination of Nutrient Composition
It is crucial to know about the nutrient content regardless of the fertilizer composition. Too much fertilizer could be distributed to plants without this information, leading to environmental pollution and undesirable fertilizer burns.
For the standardized determination of these nutrients, fertilizer producers are required to specify the number of nutrients within their products, and various norms from ISO, EN and AOAC exist.
Yet, some of the proposed analysis methods are extremely time consuming, or the techniques call for expensive equipment. For instance, phosphorus, potassium and sulfur are typically established gravimetrically or by utilizing ICP-OES.
Kjeldahl digestion is typically needed to establish nitrogen components with a subsequent acid-base titration. For the analysis of phosphorus, sulfur, potassium, ammoniacal nitrogen and urea, thermometric titration supplies an inexpensive alternative solution with no time consuming steps.
The general principle of thermometric titration is outlined below prior to describing the analysis of these specific nutrients by thermometric titration.
Principle of Thermometric Titration
Titration is an established analysis technique, where the content of a species (analyte) is ascertained by adding a reagent solution (titrant), which reacts stoichiometrically with the analyte.
The analyte content can be determined reliably by establishing the titrant volume needed for the reaction. A suitable indication technique is utilized to establish the required titrant volume.
Thermometric titration employs the principle of the reaction enthalpy to show the endpoint of the titration. Titrant and analyte react with each other either endothermically (decrease in temperature) or exothermically (increase in temperature).
The temperature in the titration vessel increases or decreases at a constant rate when the titrant is added at a constant rate. Heat is no longer consumed or generated when the endpoint of the titration is reached, and a sharp break in the temperature curve can be seen, showing the reaction endpoint (Fig. 1)3.
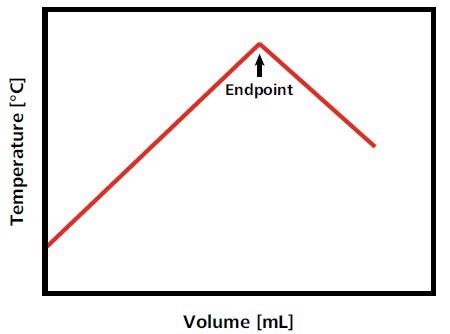
Figure 1. Idealized titration curve for an exothermic titration reaction. In this example, as long as analyte is present, the temperature increases with the titrant addition. When all analyte is consumed, the temperature decreases again as the solution equilibrates with the atmospheric temperature and/or due to the dilution of the solution with titrant. This temperature decrease results in an exothermic endpoint. Image Credit: Metrohm AG
Thermometric titrations utilize a thermistor, which can detect minute temperature changes, to determine the endpoint of a titration (Fig. 2). These sensors can measure temperature differences of below 0.001°C and enable a measuring point to be collected every 0.3 seconds.
They are almost maintenance free compared to other sensors used in titration and can be employed to determine various analytes. The applicability of thermometric titration for the analysis of numerous nutrients in fertilizers will be demonstrated in the next section.

Figure 2. The Metrohm Thermoprobe is capable of measuring temperature changes of less than 0.001°C, and allows the collection of a measuring point every 0.3 seconds. Image Credit: Metrohm AG
Applicability of Thermometric Titration for Fertilizers
Phosphorus
Phosphorus in fertilizer is typically present as phosphate. As it is a macronutrient, it is not only present in multi-nutrient fertilizers (e.g., MAP, DAP and NPK-fertilizers) but also as straight fertilizer in the form of superphosphates. Historically, total phosphorus content is quantified via gravimetric analysis.
Spectrophotometric analysis or ICP-OES may also be utilized for the determination. These techniques all require regular calibrations or time-consuming sample preparation steps.
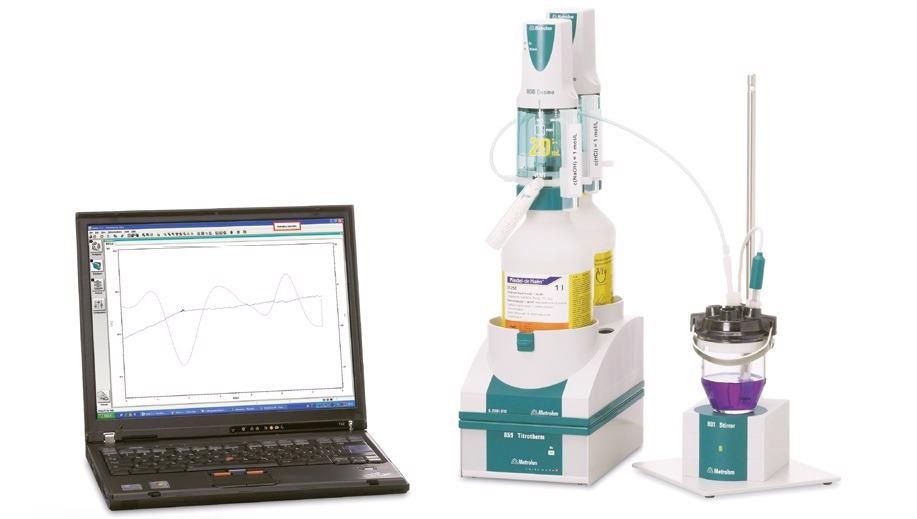
Figure 3. Thermometric titration system consisting of a Metrohm 859 Titrotherm equipped with a Thermoprobe for the indication and two 800 Dosinos for the titrant and addition of auxiliary solution. The system is controlled via the Metrohm tiamoTM software. Image Credit: Metrohm AG
The thermometric titration is based on the exothermic formation of insoluble struvite (MgNH4PO4 · 6 H2O) in alkaline media according to the following reaction equation:
Mg2+ + NH4+ + PO43- → MgNH4PO4
For the titration, magnesium nitrate is employed as a titrant in an alkaline NH3/NH4Cl buffer solution. Thus, the titration is an adoption of a classical gravimetric procedure. Results for the phosphate content can be gathered within 5 minutes when using thermometric titration, with no additional steps like filtering, washing and drying.
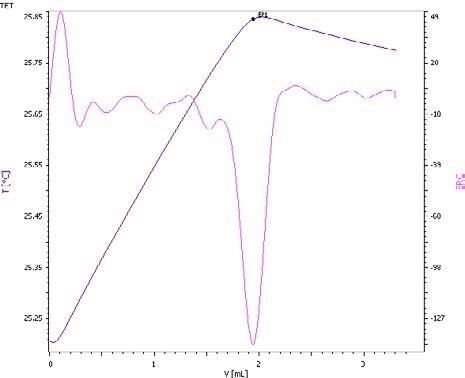
Figure 4. Exothermic titration curve of the phosphate determination in an NPK fertilizer by precipitation with magnesium ions in the presence of an ammonia/ammonium chloride buffer (blue = titration curve, pink = second derivative showing the endpoint). Image Credit: Metrohm AG
The ratio of H2PO4- and HPO42- is another crucial parameter in the phosphoric acid manufacturing process. It is not usually possible to distinguish these two species in a potentiometric acid-base titration due to the leveling effects of water.
Thermometric titration is not restricted by this problem, so it is possible to simply establish the ratio using sodium hydroxide as titrant. It becomes viable to see all three endpoints if phosphoric acid is titrated, as seen in Fig. 5.
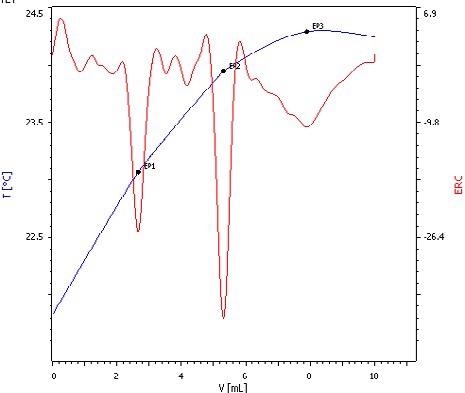
Figure 5. Exothermic titration curve of phosphoric acid titrated with NaOH. Here, all three deprotonation steps can be clearly distinguished (blue = titration curve, red = second derivative showing the endpoints). Image Credit: Metrohm AG
Potassium
As it plays a key role in water regulation as well as plant growth, potassium is a primary macronutrient for plants. It is for this reason that it is available as a straight fertilizer in the form of potash, as well as in multi-nutrient fertilizers such as potassium dihydrogen phosphate (MKP) or NPK fertilizers.
Potassium is traditionally established gravimetrically. ICP-OES or flame photometry have been used more recently. Potassium can also be determined by titration by utilizing the precipitation reaction of potassium with sodium tetraphenylborate (STPB). A reliable determination of potassium via thermometric titration produces a result in around 5 minutes.
This advantage, in addition to the minimal sample preparation and low costs per analysis, makes it the ideal solution. The technique is already incorporated into the Chinese recommended professional standard HG/T 2321 on the analysis of fertilizer grade potassium dihydrogen phosphate6.
If the fertilizer contains ammonium, it must be removed as ammonia prior to the titration, or it would precipitate concurrently with STPB and interfere with the determination.
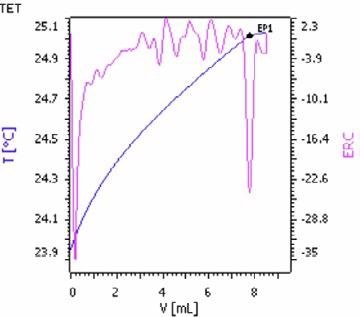
Figure 6. Exothermic titration curve of the potassium determination in potash by precipitation with STPB (blue = titration curve, pink = second derivative showing the endpoint). Image Credit: Metrohm AG
Sulfate
Sulfur is vital for chloroplast function and growth and is a secondary macronutrient for plants. Sulfur is typically supplied in the form of sulfate in fertilizers. It is also present in the wet phosphoric acid production process.
The sulfuric acid content within phosphoric acid as well as within final products (e.g., TSP, DAP, MAP and NPK fertilizers) should be known for an optimal production process.
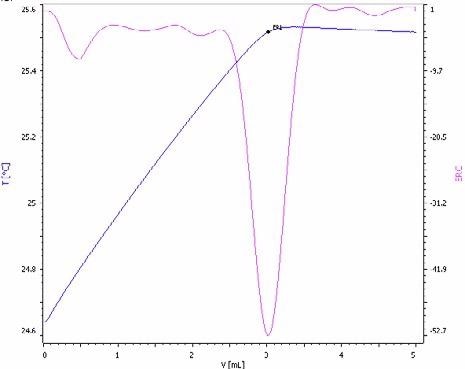
Figure 7. Exothermic titration curve of the sulfate determination in a NPK fertilizer spiked with sulfuric acid for enhanced method sensitivity (blue = titration curve, pink = second derivative showing the endpoint). Image Credit: Metrohm AG
Conventionally, sulfate content is established gravimetrically by precipitation with barium. The same reaction principle is employed for the thermometric titration and results can be gathered with only minimal sample preparation within 3 minutes.
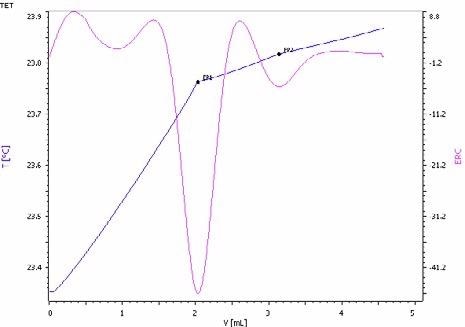
Figure 8. Exothermic titration curve of the ammoniacal nitrogen and urea determination in a NPK fertilizer. The first endpoint corresponds to the ammonia and the second to urea (blue = titration curve, pink = second derivative showing the endpoint). Image Credit: Metrohm AG
The samples can be spiked with a standard sulfuric acid solution in order to enhance the sensitivity of the technique, which is then considered when calculating the result. Calcium (e.g., from CAN fertilizers) can interfere with this determination as it can form insoluble calcium sulfate. So it must either be precipitated with excess oxalate or removed prior to the titration using cationic exchange resins.
Ammoniacal Nitrogen and Urea
The third significant macronutrient which plants require is nitrogen. This represents the largest product group of fertilizers by far. Nitrogen in fertilizers can be present in numerous forms, including nitrate, ammonium, or urea.
Ammonia is typically determined after alkaline distillation by acid-base back-titration, while other nitrogen species are usually first converted to ammonia by digestion prior to analysis. A different method is employed to determine ammonia content for the thermometric titration.
Ammonium ions react exothermically with hypochlorite in a redox reaction. This reaction is catalyzed further in the presence of bromide ions in a slightly alkaline solution, where the more reactive species hypobromite is formed, which then reacts with ammonium to form nitrogen.
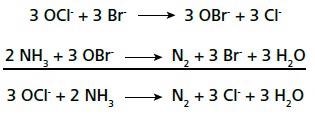
This titration method provides results after an analysis time of 2 minutes without any prior distillation step. Urea has the highest nitrogen concentration among straight fertilizers, which contain nitrogen.
Urea is generated from carbon dioxide and ammonia. The production process is affected by the amount of ammonia present, so during the process, it is necessary to know the ratio of urea and ammonia present7.
It is possible to determine ammoniacal nitrogen in the sample and also the urea by using the aforementioned titration method for ammoniacal nitrogen. This is because urea also reacts with hypobromite, but with a slower reaction rate.
The separation of ammonium and urea content in a single titration becomes possible by utilizing an appropriate dosing rate of titrant. The fertilizer can be spiked with some urea for a better separation of both endpoints.
Thermometric Titration: The Ideal Solution to Analyze Fertilizers
Thermometric titration is an inexpensive alternative technique to analyze the key substances within fertilizers or during their production processes. Time consuming sample preparation and analysis steps can also be left out, allowing analysts to gather results within minutes.
This enables plant operators to optimize process settings much faster. It greatly enhances the efficiency of the production plants while at the same time liberating chemists and lab technicians to perform other tasks
References
- Our World in Data. World population with and without synthetic nitrogen fertilizers. https://ourworldindata.org/grapher/world-population-with-and-without-fertilizer?time=1900..2015 (Accessed March 23, 2020).
- ISO 6598:1985 Fertilizers – Determination of phosphorus content — Quinoline phosphomolybdate gravimetric method. Geneva: International Organization for Standardization, 1985. https://www.iso.org/standard/13009.html
- ISO 17319:2015 Fertilizers and soil conditioners – Determination of water-soluble potassium content — Potassium tetraphenylborate gravimetric method. Geneva: International Organization of Standardization, 2015. https://www.iso.org/standard/59569.html
- ISO 10084:1992 Solid fertilizers – Determination of mineral-acid-soluble sulfate content — Gravimetric method. Geneva: International Organization for Standardization, 1992. https://www.iso.org/standard/18056.html
- ISO/CD 20917 Determination of Available Phosphorous and Soluble Potassium Extracted with Neutral Ammonium Citrate and Quantified by ICP-OES. Geneva: International Organization for Standardization, 2020. https://www.iso.org/standard/69457.html
- Fertilizers Europe. Types of fertilizer. https://www.fertilizerseurope.com/fertilizers-in-europe/types-of-fertilizer/ (Accessed May 7, 2020).
- Smith, T. Practical thermometric titrimetry; Metrohm Monograph: Herisau, Switzerland, 2006. https://www.metrohm.com/en/documents/80365003
- Fertilizers Europe. Booklet - BAT Production of phosphoric acid. https://www.fertilizerseurope.com/wp-content/uploads/2019/08/Booklet_4_final.pdf (Accessed May 7, 2020).
- Bache, S. Rapid phos acid process monitoring. Fertilizer International. Issue 486, September-October 2018.
- HG/T 2321-2016 Fertilizer grade potassium dihydrogen phosphate. Beijing: The Standardization Administration of the People's Republic of China, 2016.
- Fertilizers Europe. Booklet - BAT Production of urea and urea ammonium nitrate. https://www.fertilizerseurope.com/wp-content/uploads/2019/08/Booklet_5_final.pdf (Accessed May 7, 2020).
- Fertilizers Europe. How are fertilizers made? https://www.fertilizerseurope.com/fertilizers-in-europe/how-fertilizers-are-made/ (Accessed May 7, 2020).

This information has been sourced, reviewed and adapted from materials provided by Metrohm AG.
For more information on this source, please visit Metrohm AG.