The addition of unauthorized materials into food products has been a key concern amongst consumers in recent years. For instance, in Islam, the consumption of pork and/or products made from pork is strictly forbidden.
So, it is vital to develop a method that can identify and quantify the biomarkers which are specific for pork meat and pork related products.1,2
A sensitive and selective LC/MS/MS technique has been developed using three peptides specific for pork as biomarkers for the quick identification and analysis of meat samples.
Experimental
Sample Preparation
Meat products or ground meats weighing 2 g were dissolved in 5 mL of extraction solution.1-4 Samples were vortexed and centrifuged for 10 min with 12000 rpm/min.
After being cooled to room temperature, 0.5 mL of supernatant was reduced by dithiothreitol, alkylated with iodoacetamide in a dark place, diluted with 800 µL of 25 mmol/L Tris-HCl (pH 8.0) and supplemented with sequencing grade modified trypsin.
Samples were incubated in a thermos-shaker at 37 °C under slow shaking overnight to enable total digestion. The enzymatic activity was quenched with 20 µL of formic acid.
SPE cartridges were used to desalt and concentrate digested samples. To reduce the volume, the SPE eluents containing the peptides were dried and then filtered through a 0.22µm membrane filter before LC/MS/MS analysis.
LC Separation
A PerkinElmer QSight® LX-50 UHPLC system was used to separate the analytes on a C18 column.
The temperature of the column oven was set at 40 °C. The mobile phases consisted of water (A) and acetonitrile (B), both containing 0.1 % formic acid. The injection volume was 10 μL. The elution gradient is shown in Table 1, and the flow rate was 0.4 mL/min.
Table 1. LC eluent gradient. Source: PerkinElmer Food Safety and Quality
| Time (min) |
Flow Rate (mL/min) |
A (%) |
B (%) |
| 0 |
0.4 |
98 |
2 |
| 0.8 |
0.4 |
98 |
2 |
| 14 |
0.4 |
60 |
40 |
| 15 |
0.4 |
2 |
98 |
| 17.5 |
0.4 |
2 |
98 |
| 17.6 |
0.4 |
98 |
2 |
| 20 |
0.4 |
98 |
2 |
MS Conditions
The QSight LX-50 UHPLC system was coupled to a QSight 220 triple quadrupole mass spectrometer equipped with an electrospray ionization source working in positive ion mode.
The mass spectrometer operating conditions were as follows:
- Nebulizer Gas: 180
- Dry Gas: 100
- ElectroSpray Voltage: 5000
- HSID Temp: 320 °C
- Heating Gas Temp: 400 °C
Detection of analytes by tandem mass spectrometry was performed in multiple reaction monitoring mode (MRM). Three specific peptides, each with 2 MRM transitions, were utilized to analyze samples in a single injection. Data processing and acquisition were carried out using PerkinElmer Simplicity™ 3Q software.
Results and Discussion
The specificity of the three selected peptides based on their unique MRM transitions in pork was tested in a raw pork matrix, as seen in Figure 1, and compared with other meat matrices like chicken, lamb, beef and duck, as seen in Figure 2.
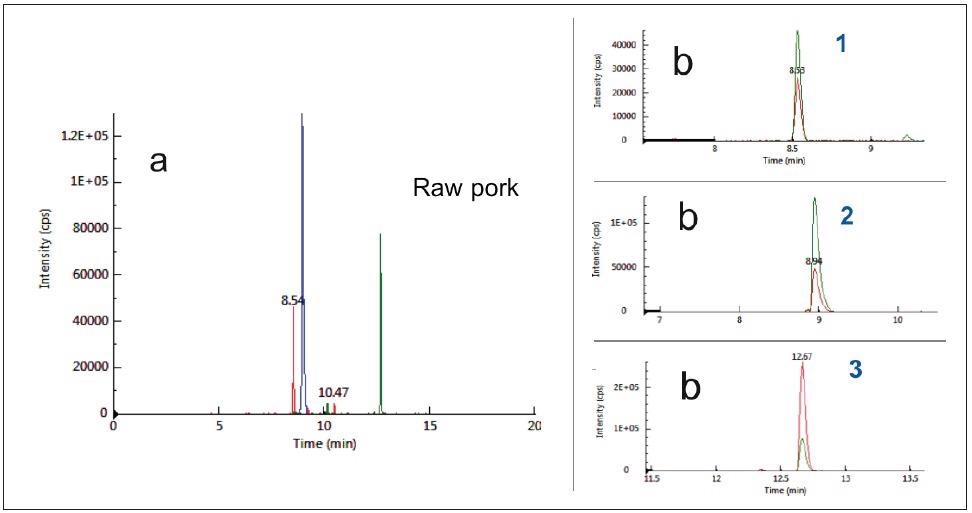
Figure 1. Typical chromatograms of raw pork as acquired by MRM mode: (a) Total ion chromatograms (b) Extract ion chromatograms. Notes: 1:Pork_Peptide_1, Rt 8.5 min; 2:Pork_Peptide_2, Rt 8.9 min; 3:Pork_Peptide_3 , Rt 12.7min. Image Credit: PerkinElmer Food Safety and Quality
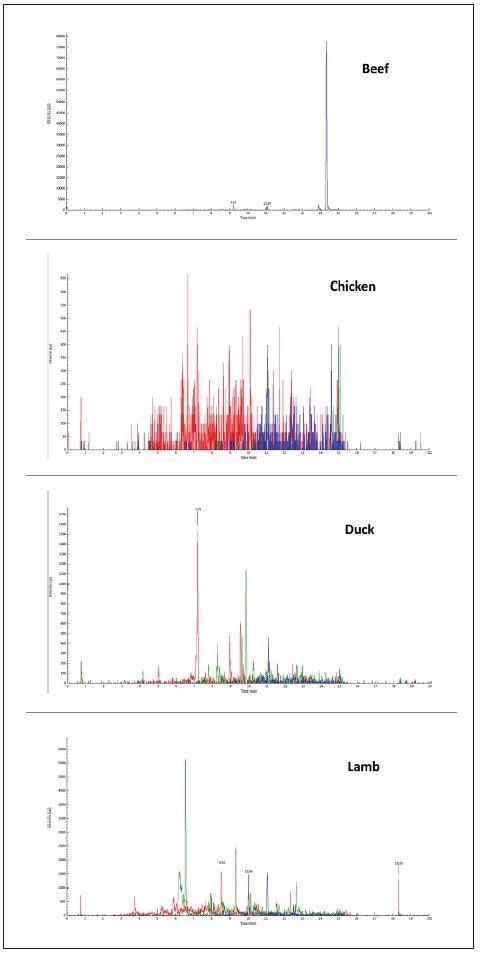
Figure 2. Typical chromatograms of other meat matrices as acquired by MRM mode: chicken, duck, lamb, beef. Image Credit: PerkinElmer Food Safety and Quality
The results indicated that the three peptides could be utilized as biomarkers for pork and pork-originated products as they were unique and specific for pork. By testing their MRM responses in raw and cooked pork (hard-boiled) the thermal stability of the biomarker peptides was evaluated.
Each peptide was detected without significant changes in sensitivity both before and after cooking, demonstrating their thermal stability, as shown in Figure 3.
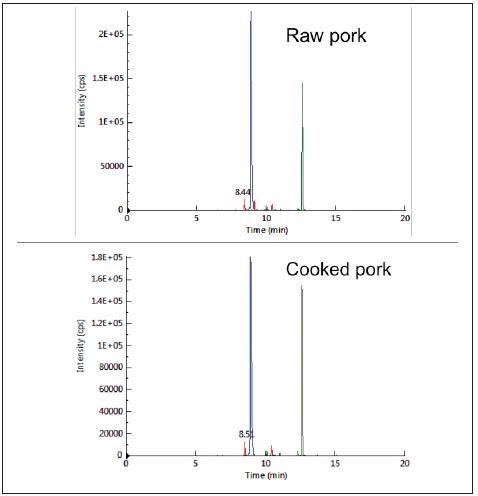
Figure 3. Typical chromatograms from LC-MRM analysis of raw (top) and cooked (bottom) pork. Image Credit: PerkinElmer Food Safety and Quality
For each of the three peptides, calibration curves were produced over a wide concentration range (1 to 100 % w/w) with a good linear response in the pork sample, as seen in Figure 4.
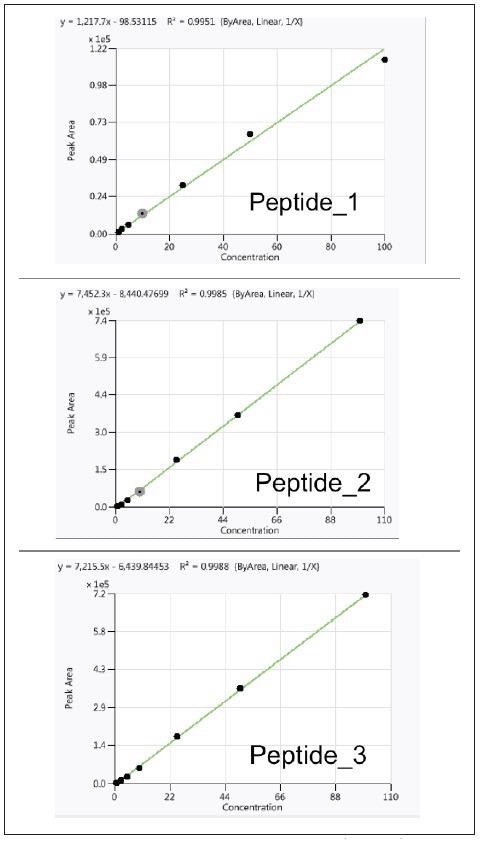
Figure 4. Calibration curves for three peptides in pork matrix (R2>0.990). Image Credit: PerkinElmer Food Safety and Quality
Limits of quantification (LOQ) values for this technique are 1 % (w/w) spiked pork in the meat mixture.
Conclusions
A sensitive, fast and selective LC/MS/MS technique has been developed for the analysis of three peptides in cooked and raw pork meat. The optimized sample preparation procedure can be used for analyzing raw, cooked and processed meat products and is easy to follow.
In a variety of food products, this technique is able to selectively detect peptides from pork meat species at a threshold detection limit of 1 % w/w (10 mg/g). Therefore, the technique can be useful for the analysis of Halal food (or pork related food products) samples.
References
- Christoph v B, Jörg n, Dietmar W, et al. J. Agric. Food Chem. 2013, 61, 11986−11994.
- Christoph v B, Jens B, Hans-Ulrich H, et al. J. Agric. Food Chem. 2014, 62, 9428−9435.
- Andrew D. W, Yvonne G, Neil M. R, et al. Anal. Chem. 2015, 87, 10315−10322.
- Yvonne G, Andrew D. W, Neil M. R, et al. J. Visu. Expert. 2016, 115, e54420, 1-7.

This information has been sourced, reviewed and adapted from materials provided by PerkinElmer Food Safety and Quality.
For more information on this source, please visit PerkinElmer Food Safety and Quality.