One of the primary challenges associated with electrochemical carbon dioxide reduction reaction (eCO2RR) is the need to employ an active catalyst able to produce hydrocarbons that exhibit a high current density and a relatively low overpotential.
A recent study published in Nature Communications by Professor Asadi and his research team at Illinois Tech showcased a new class of catalysts able to effectively convert CO2 to a range of hydrocarbons, including ethylene (C2H4), methane (CH4), methanol (CH3OH) and ethanol (C2H5OH).
These resulting hydrocarbons displayed exceptionally high current density (reaction rate) and faradaic efficiency (selectivity), demonstrating better performance than gold in terms of activity and better performance than copper in terms of selectivity. Both gold and copper are regarded as state-of-the-art catalysts for eCO2RR.
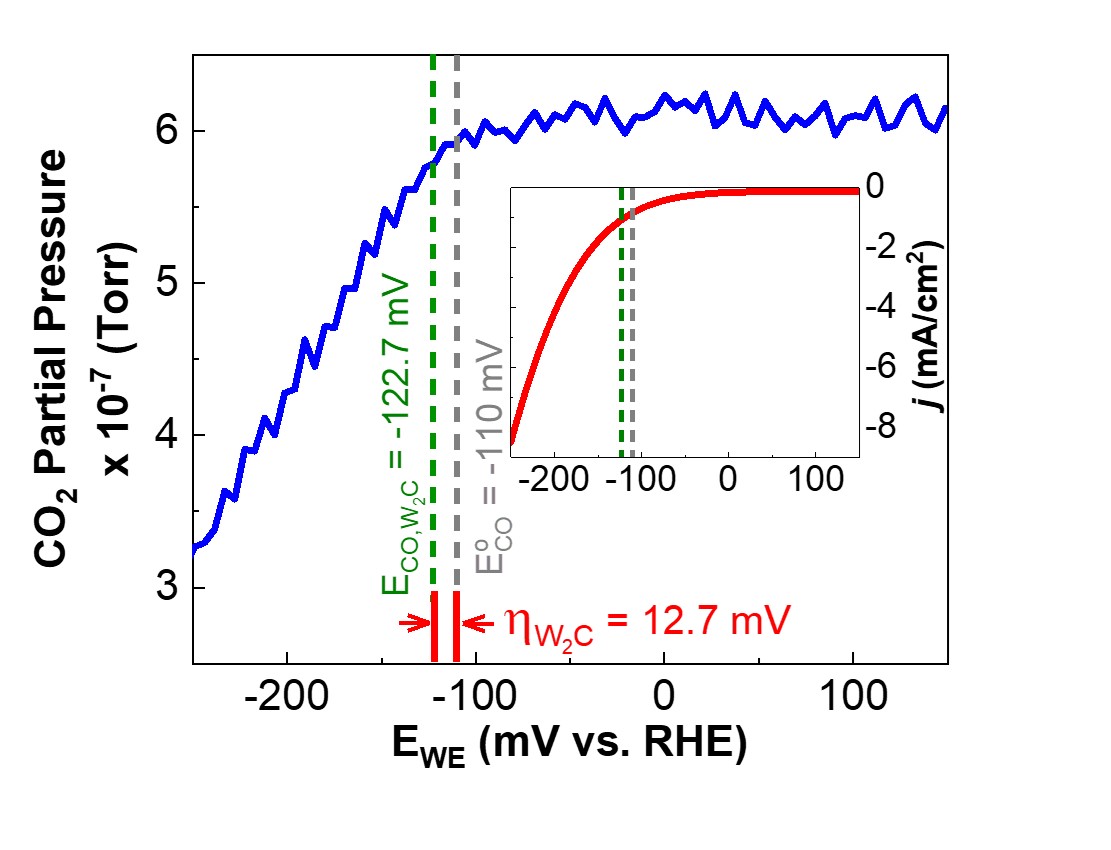 The CO2 partial pressure as a function of potential and corresponding LSV result (inset) are shown for W2C nanoflakes as the catalyst for eCO2RR. Image Credit: Hiden Analytical
The CO2 partial pressure as a function of potential and corresponding LSV result (inset) are shown for W2C nanoflakes as the catalyst for eCO2RR. Image Credit: Hiden Analytical
Professor Asadi and his team have developed a zero-gap flow electrolyzer for eCO2RR. This electrolyzer uses di-tungsten carbide (W2C) nanoflakes as the cathode catalyst and is able to deliver long-term stability of 700 hours, a current density of 548.89 mA/cm2 at 2.3 V, and CH4 selectivity of 82.7.
Real-time differential electrochemical mass spectroscopy (Hiden Analytcial HPR-40-DEMS) was used to confirm a CO2RR onset potential of 12.7 mV.
A combined computational and experimental study was conducted in collaboration with scientists at Molecular Foundry, Lawrence Berkeley National Laboratory.
This study highlighted that the superior electrocatalytic performance of the W2C catalyst led to almost spontaneous chemisorption of CO2 and cleavage of the C-O bond at the tungsten surface atoms.
References
- “Gold-like activity copper-like selectivity of heteroatomic transition metal carbides for electrocatalytic carbon dioxide reduction reaction” Nature Communications (2021) 12, 5067 doi.org/10.1038/s41467- 021-25295-y
Acknowledgments
Produced from materials originally authored by Mohammad Asadi from the Department of Chemical and Biological Engineering, Illinois Institute of Technology.

This information has been sourced, reviewed and adapted from materials provided by Hiden Analytical.
For more information on this source, please visit Hiden Analytical.