This article is based on a poster originally authored by Oliver Buettel.
Various metals can negatively affect production processes and final product performances, requiring their analysis in Naphtha.
Volatile organic solvents are associated with plasma destabilization, which can be caused by them. Organic matrices can cause carbon- and plasma-based polyatomic interference, resulting in carbon deposits on the torch and interface cones, creating signal drift. To improve product specifications, the required detection limits are exceptionally low.
The Naphtha samples were diluted in n-Hexane (1:10). MDL accounts for the dilution factor.
Table 1. PlasmaQuant MS settings and method parameters for naphtha analysis. Source: Analytik Jena US
| Parameter |
Specification |
Parameter |
Specification |
| Plasma gas flow |
10.5 L/min |
Pump rate (drain spray chamber) |
30 rpm - black/black Viton® pump tubing |
| Auxillary gas flow |
1.50 L/min |
Stabilization delay |
20 s |
| Sheath gas flow |
0.50 L/min |
iCRC |
H2 - 200 mL/min |
| Nebulizer gas flow |
0.40 L/min |
Nitrox |
02 - 180 mL/min |
| Plasma RF power |
1.30 kW |
Skimmer Bias (BOOST) |
10 V |
| Nebulizer type |
MicroMistTM 0.4 mL/min (quartz concentric) |
Dwell time |
20 ms |
| Cones |
Platinum |
Scan mode |
peak hopping, 1 pt/peak |
| Torch |
Fassel torch with 1.5 mm injector |
No. of scans per replicate |
25 |
| Spray chamber type and temperature |
Glass Scott with Peltier chiller, -10 °C |
No. of replicates per sample |
5 |
| Sample introduction |
Self aspiration |
Acquisition time |
140 s |

 Download the Poster
Download the Poster
Figures of Merit
- Precision and accuracy:
- Spike recovery (10 ng/g): 90-110% for all elements
- Duplicate repeatability (2.54 ng/g):
- Mg: 1.42%
- Fe: 0.70%
- Pb: 0.86%
- Extended stability (4 hours):
- QC Standard (2.54 ng/g): < 10% deviation
- Sample replicate analysis: < 5% RSD
- Internal Standard (Y, 25 ng/g): 7% drift
Patented Analytik Jena Technology
iCRC with BOOST Technology
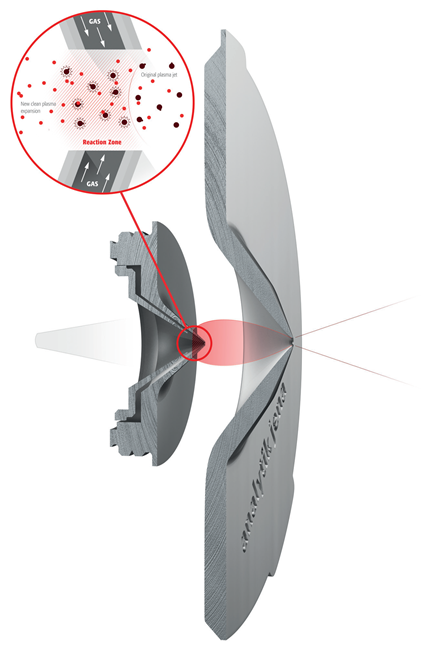
Image Credit: Analytik Jena US
- Collision-Reaction-Cell integration with the cone interface
- Simplified and effective removal of polyatomic interferences
- H2 / He injection through skimmer cone
- Extra consumables and maintenance are not required
- BOOST: Skimmer bias voltage retrieves sensitivity loss in gas-modes
ReflexION Ion Mirror
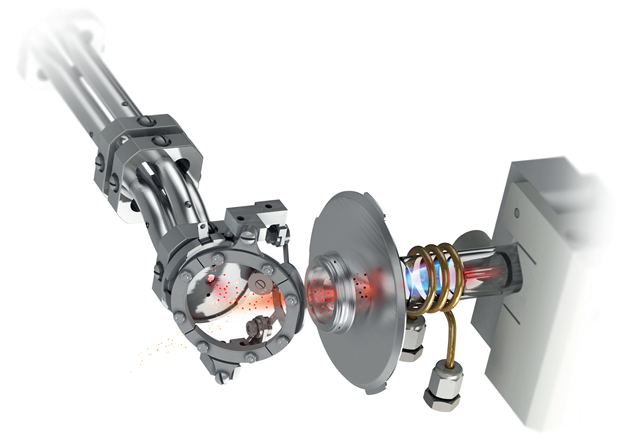
Image Credit: Analytik Jena US
- Designed with the original 90° ion optical
- The electrostatic field functions as a parabolic mirror.
- It reflects and focuses the ion beam into the mass analyzer.
- An industry leader with exceptional sensitivity and transport efficiency.
- There is no required maintenance or use of consumables.
Table 2. Method detection limits in blank solvent (ng/g). Source: Analytik Jena US
| Isotope |
MDL |
Isotope |
MDL |
Isotope |
MDL |
Isotope |
MDL |
| 10B |
3.1 |
52Cr |
0.03 |
66Zn |
0.30 |
118Sn |
0.02 |
| 24Mg |
1.1 |
55Mn |
0.09 |
75As |
0.06 |
137Ba |
0.04 |
| 49Ti |
1.3 |
56Fe |
0.25 |
98Mo |
0.02 |
200Hg |
0.04 |
| 51V |
0.11 |
60Ni |
0.65 |
114Cd |
0.01 |
206+207+208Pb |
0.01 |
Conclusion
PlasmaQuant MS has exhibited an exceptional ability to analyze trace metals in Naphtha and other volatile solvents. This method has exceeded all the requirements of ASTM D8110-17. iCRC H2-mode effectively eliminates polyatomic interferences with high sensitive detection limits.
Robust plasma provides precision and long-term stability. It is user-friendly and easy to maintain and has proven performance for even the most difficult samples.
PlasmaQuant MS is engineered for excellence.

 Download the Poster
Download the Poster

This information has been sourced, reviewed and adapted from materials provided by Analytik Jena US.
For more information on this source, please visit Analytik Jena US.