The determination of diethylene glycol content, isophthalic acid content, intrinsic viscosity (ASTM D4603), and acid number (AN) in polyethylene terephthalate (PET) is a lengthy and challenging process due to the sample's limited solubility and the requirement to use multiple analytical methods.
In the study presented in this article, the DS2500 Solid Analyzer operating in the visible and near-infrared spectral region (Vis-NIR) is demonstrated as a cost-efficient and rapid solution for simultaneous measurement of diethylene glycol and isophthalic acid content, intrinsic viscosity, and AN of PET.
Vis-NIR spectroscopy allows for the analysis of PET in under one minute without the need for sample preparation or chemical reagents.
Equipment
PET pellets were measured using a DS2500 Solid Analyzer in reflection mode over the full wavelength range (400–2500 nm).
A rotating DS2500 Large Sample Cup was utilized to address the distribution of diverse particle sizes and chemical components. This cup allowed for automated measurements at different sample locations and ensured reproducible spectrum acquisition.
Samples were measured without a preparation step, as shown in Figure 1. The Metrohm software package Vision Air Complete was employed for all data acquisition and prediction model development.

Figure 1. DS2500 Solid Analyzer with PET pellets present in the rotating DS2500 Large Sample Cup. Image Credit: Metrohm Middle East FZC
Table 1. Hardware and software equipment overview. Source: Metrohm Middle East FZC
| Equipment |
Metrohm number |
| DS2500 Solid Analyzer |
2.922.0010 |
| DS2500 Large Sample Cup |
6.7402.050 |
| Vision Air 2.0 Complete |
6.6072.208 |
Results
The Vis-NIR spectra obtained (Figure 2) were utilized to develop prediction models for quantifying diethylene glycol, isophthalic acid, intrinsic viscosity, and acid number.
The quality of these prediction models was assessed using correlation diagrams that illustrate the relationship between Vis-NIR predictions and values obtained from primary methods. The respective figures of merit (FOM) indicate the expected precision of these predictions during routine analysis.
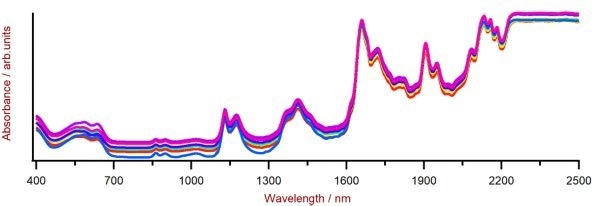
Figure 2. Selection of PET Vis-NIR spectra obtained using a DS2500 Analyzer and a rotating DS2500 Large Sample Cup. For display reasons a spectra offset was applied. Image Credit: Metrohm Middle East FZC
Diethylene Glycol Content
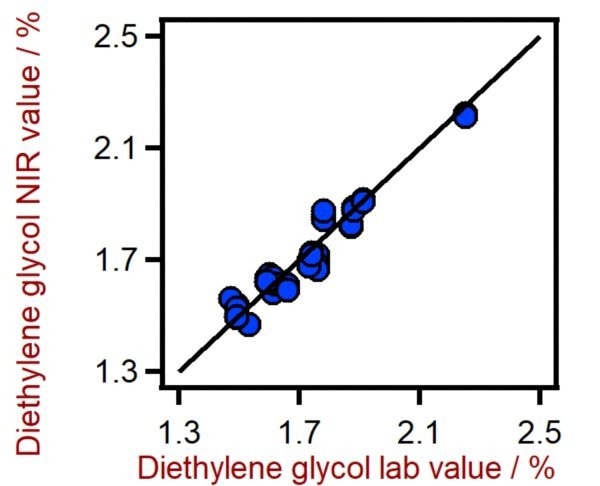
Figure 3. Correlation diagram for the prediction of the diethylene glycol content in PET using a DS2500 Solid Analyzer. The diethylene glycol lab value was evaluated using HPLC-MS. Image Credit: Metrohm Middle East FZC
Table 2. Figures of merit for the prediction of the diethylene glycol content in PET using a DS2500 Solid Analyzer. Source: Metrohm Middle East FZC
| Figures of merit |
Value |
| R2 |
0.931 |
| Standard error of calibration |
0.052 % |
| Standard error of cross-validation |
0.066 % |
Isophthalic Acid Content
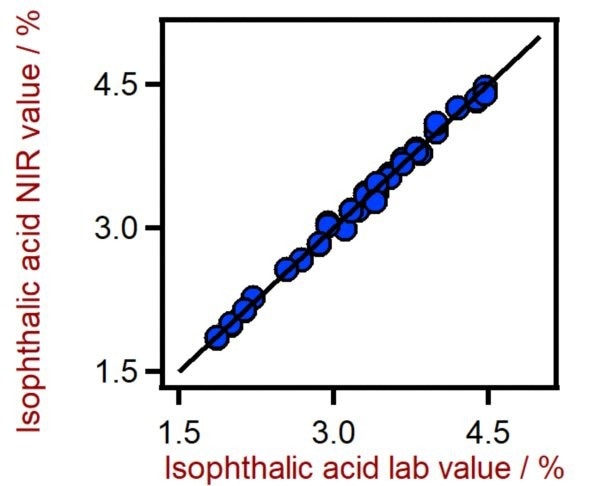
Figure 4. Correlation diagram for the prediction of the isophthalic acid content in PET using a DS2500 Solid Analyzer. The isophthalic acid lab value was evaluated using HPLC. Image Credit: Metrohm Middle East FZC
Table 3. Figures of merit for the prediction of the isophthalic acid content in PET using a DS2500 Solid Analyzer. Source: Metrohm Middle East FZC
| Figures of merit |
Value |
| R2 |
0.995 |
| Standard error of calibration |
0.059 % |
| Standard error of cross-validation |
0.085 % |
Intrinsic Velocity
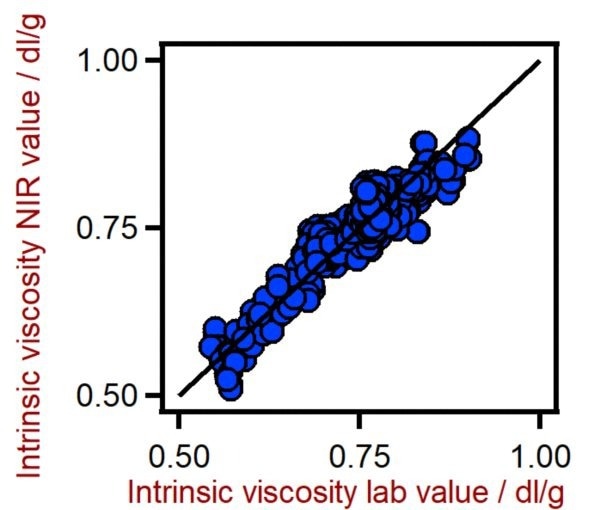
Figure 5. Correlation diagram for the prediction of the intrinsic viscosity of PET using a DS2500 Solid Analyzer. The intrinsic viscosity lab value was evaluated using viscometry. Image Credit: Metrohm Middle East FZC
Table 4. Figures of merit for the prediction of the intrinsic viscosity of PET using a DS2500 Solid Analyzer. Source: Metrohm Middle East FZC
| Figures of merit |
Value |
| R2 |
0.873 |
| Standard error of calibration |
0.0236 |
| Standard error of cross-validation |
0.0238 |
Acid Value
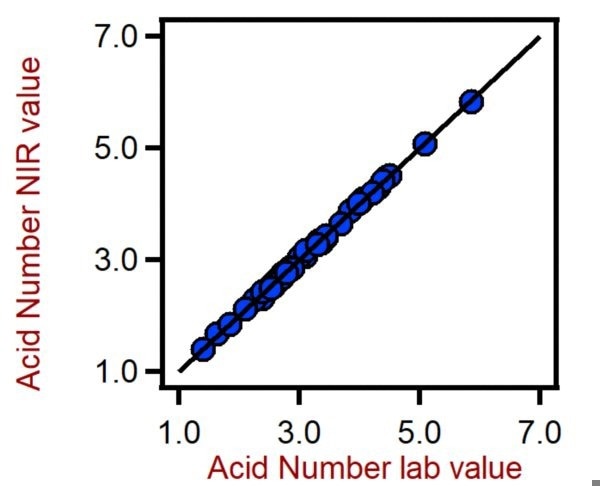
Figure 6. Correlation diagram for the prediction of the acid number in PET using a DS2500 Solid Analyzer. The Acid Number lab value was evaluated using titration. Image Credit: Metrohm Middle East FZC
Table 5. Figures of merit for the prediction of the acid number in PET using a DS2500 Solid Analyzer. Source: Metrohm Middle East FZC
| Figures of merit |
Value |
| R2 |
0.991 |
| Standard error of calibration |
0.093 |
| Standard error of cross-validation |
0.143 |
Conclusions
The study presented here demonstrates the viability of NIR spectroscopy for the analysis of key PET quality parameters. Compared to wet chemical methods (see Table 6), speed of measurement is a key advantage of NIR spectroscopy, as all parameters are determined in a single measurement in less than one minute.
Table 6. Time to result overview for the different parameters. Source: Metrohm Middle East FZC
| Parameter |
Method |
Time to result |
| Diethylene glycol |
Extraction + analysis HPLC-MS |
∼45 min (preparation) + ∼40 min (HPLC) |
| Isophthalic acid |
Dissolve + HPLC |
∼45 min (preparation) + ∼40 min (HPLC) |
| Intrinsic viscosity |
Dissolve + viscometry |
∼90 min (preparation) + ∼1 min (viscometry) |
| Acid Number |
Dissolve + titration |
∼90 min (preparation) + ∼10 min (titration) |

This information has been sourced, reviewed and adapted from materials provided by Metrohm Middle East FZC.
For more information on this source, please visit Metrohm Middle East FZC.