X-ray photoelectron spectroscopy (XPS) is a powerful surface-sensitive analytical technique ideally suited to the characterization of chemical composition and interfacial interactions within the multilayer packaging materials found in potato chip packets.
These packets comprise laminated layers, including polypropylene (PP), polyethylene (PE), and metallized films such as aluminum-coated PET.
XPS facilitates the identification of elemental constituents and their respective chemical states within each layer’s top few nanometers, providing valuable insights into barrier performance, adhesion quality, and degradation mechanisms.
Analyzing the laminate’s surface and cross-sectional profiles via XPS reveals the migration of additives, the presence of oxidation, and any potential delamination zones. These characteristics are key to the optimization of packaging recyclability and durability.

Image Credit: HCavid/Shutterstock.com
Contemporary chip Packet Materials
Recent years have seen potato chip packaging grow in complexity. Multilayer materials are now widely used to ensure freshness, protect the product against oxygen and moisture, and ensure packaging durability. Several common layers are present in the packaging material.
Outer Layer (Print Layer)
This layer typically comprises oriented polypropylene (OPP) or polyester (PET). Its role is to allow printability for product information and branding, as well as offering a gloss finish and mechanical strength.
Barrier Layer
This layer includes a metallized film, such as metallized PET or OPP, EVOH (ethylene vinyl alcohol), or aluminum foil. It protects the contents from light, oxygen, and moisture, preserving flavor and chipness.
Inner Layer (Sealant Layer)
This layer typically features cast polypropylene (CPP) or low-density polyethylene (LDPE). It allows heat sealability and maintains food contact safety by ensuring the package can be tightly sealed.
Optional Layers
chip packets may also feature an adhesive layer, allowing different layers to be bonded together. This layer is frequently comprised of polyurethane or other laminating adhesives.
Anti-static or slip agents may also be added to chip packets to enhance handling and machinability.
The lamination process ensures that chip packet layers remain bonded during storage but exhibit predictable behavior once opened. Manufacturers must ensure a careful balance between seal strength and tear resistance to ensure protection alongside ease of opening.
The generation of microplastics when opening plastic packaging is also facing increasing scrutiny.1
The experiment shown here leveraged XPS in the characterization of the opened, cleaved seal of a commercially manufactured chip packet. The tear caused by opening typically follows an uneven or zigzag path as a result of the varying mechanical properties of each layer.
Results
A potato chip packet’s opened, previously sealed region was mounted using non-conductive double-sided tape.
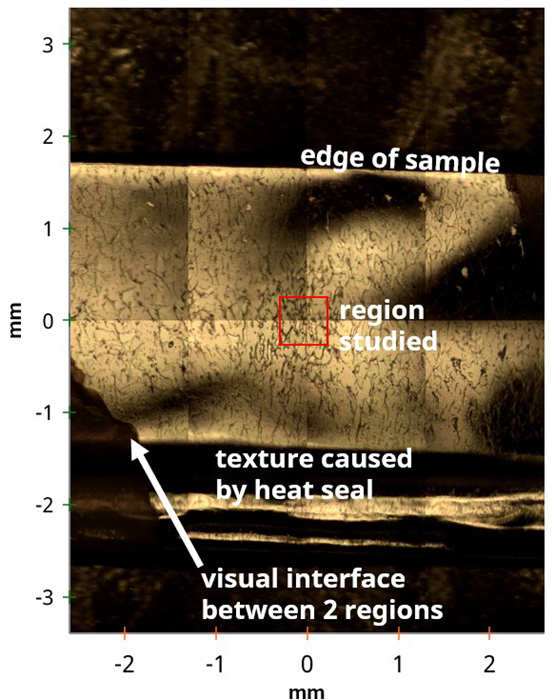
Figure 1. Stitched 4x4 optical image of an opened chip packet sample. Image Credit: Kratos Analytical, Ltd.
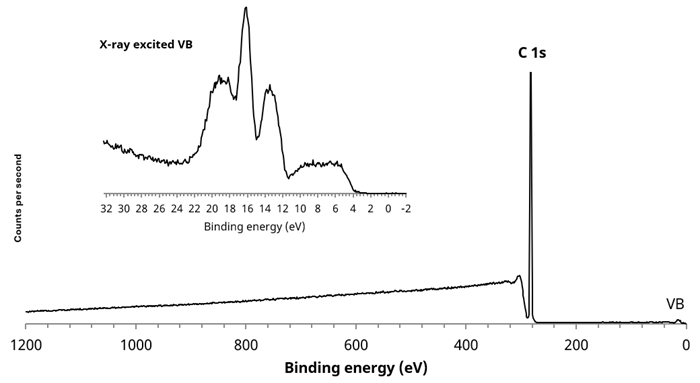
Figure 2. Large area (700 x 300 µm) survey spectrum acquired from white polymer area. Inset, X-ray excited VB spectrum. Image Credit: Kratos Analytical, Ltd.
XPS data was acquired using monochromated Al Kα X-rays with standard, electron-only charge neutralization. Survey spectra were acquired using a pass energy of 160 eV, while narrow region scans were acquired using a 40 eV pass energy.
Figure 1 shows an optical image highlighting several areas of interest. For example, a large area survey spectrum of the homogeneous white region revealed a simple, clean C 1s spectrum, which is indicative of PE or PP.
It was not possible to identify the specific polymer via the high-resolution C 1s spectrum due to carbon being in the same chemical environment for both polymers. However, the X-ray excited valence band spectrum for each polymer is notably different, enabling qualitative identification by comparing the polymer to reference spectra for each material.2
Using this method, it was clear that the valence band spectrum acquired from the white polymer region was that of polypropylene.
The area with a ‘crazed’ structure highlighted in Figure 1 was also of great interest. Figure 3 features a large area survey spectrum for this region.
Aluminum was observed in this spectrum, suggesting that the measurement includes the barrier layer, which is made up of Al-metallized poly(ethylene terephthalate) or polypropylene. XPS images were acquired to further characterize this area, enabling an investigation into any lateral distribution suggested by the optical microscope image.
Figure 4 features a stitched image acquired by combining fixed field of view parallel images with calibrated stage movements. This features the Al distribution, showing a number of discrete 100-200 µm Al features across the 2 mm x 1.3 mm image.
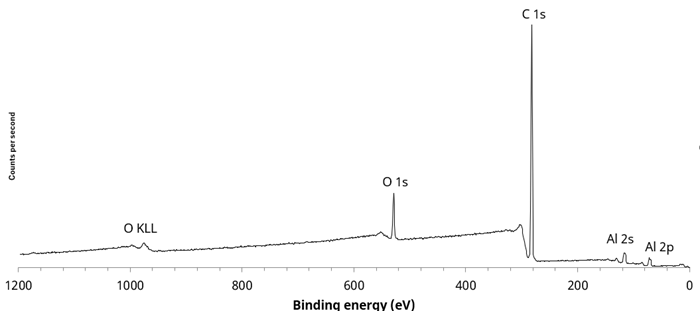
Figure 3. Large area survey spectrum taken from ‘crazed’ textured area. Image Credit: Kratos Analytical, Ltd.
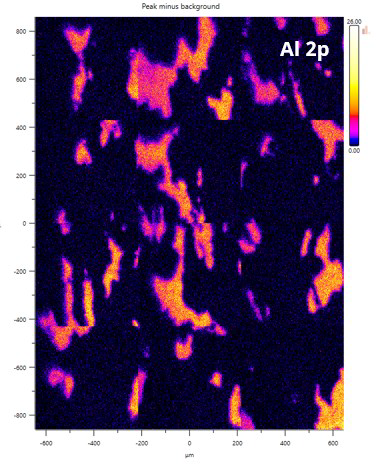
Figure 4. A 3 x 4 stitched image showing Al (peak-background) distribution. Image Credit: Kratos Analytical, Ltd.
It was possible to acquire quantitative images for aluminum, carbon, and oxygen by integrating the 2D distribution corresponding to the peak maximum and the background to the lower binding energy side of the peak for each core level before dividing the peak minus background image by the total (sum) of all three images.
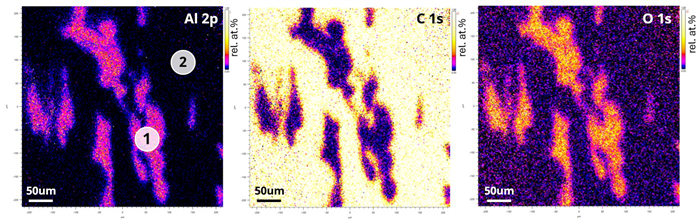
Figure 5. Quantitative FoV2 (450 µm2) XPS images showing aluminium, carbon and oxygen distribution. Brighter colours indicate higher concentration. Image Credit: Kratos Analytical, Ltd.
The quantitative images displayed in Figure 5 clearly show that the oxygen observed is linked to the aluminum present.
Next, 55 µm diameter selected area spectra were acquired from two discrete locations to further investigate the inhomogeneity of the surface. These spectra are shown on the Al 2p image in Figure 2.
Figure 6 features an overlay of the two survey spectra. It was observed that the Al 2p peak displays a double component, reflected in the broadened Al 2s peak.
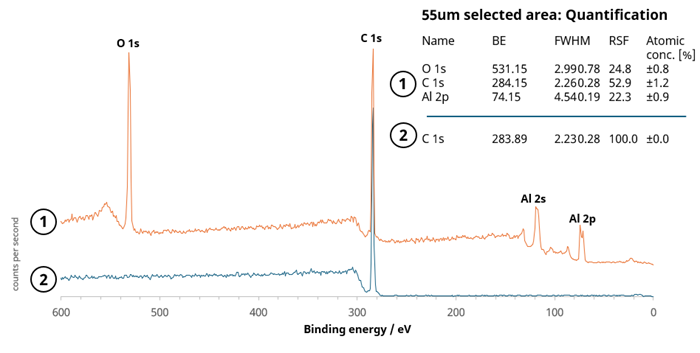
Figure 6. Overlay of 55 µm survey spectra from 2 analysis positions indicated in Figure 5. The quantification for each spectrum is also shown. Image Credit: Kratos Analytical, Ltd.
Figure 7 shows the aluminum chemistry that had been determined via a 20 eV pass energy, high-resolution, narrow region scan. The spectrum shown confirms the presence of aluminum in oxide and elemental form, potentially indicating a thin alumina passivation layer on top of the metallic thin film.
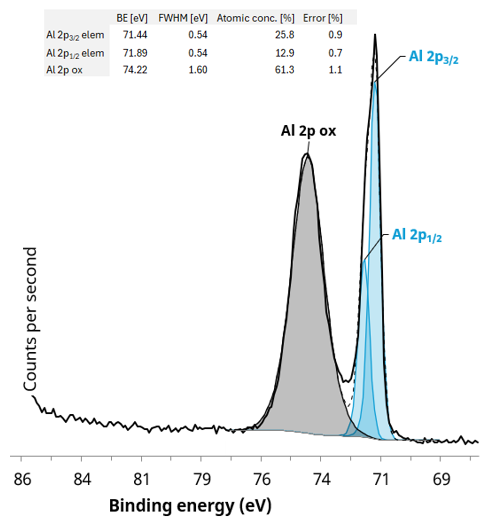
Figure 7. Al 2p region from a 55 µm selected area with quantification of the fitted Al 2p components. Image Credit: Kratos Analytical, Ltd.
The oxide passivation layer was determined to be ca. 3 nm. This was done using the method defined by Strohmeier3 and Carlson4 for the calculation of a uniform overlayer from the oxide and metallic Al 2p peak’s experimental intensity ratio. It is assumed that the formation of this passivation layer occurs during the metallization process of the polymer film utilized as the barrier layer.
The opened chip packet seal’s inhomogeneity offers useful information on the multilayer materials’ mechanical behavior during opening. The barrier layer in this material will be brittle versus the plastics, often cracking or delaminating as the outer layer is torn.
The behavior during opening is carefully engineered, with the lamination process ensuring that the layers remain bonded during storage while exhibiting predictable behavior during opening. It is important that seal strength and tear resistance are balanced with ease of opening.
The survey spectrum from Position 2 confirms that the material is extremely clean and only comprised of carbon, suggesting that the polymer material is polyethylene or polypropylene as opposed to the anticipated poly(ethylene terephthalate).
The X-ray excited valence band spectrum was recorded and compared to reference spectra to unambiguously identify the type of polymer present.2
Figure 8 features the valence band spectrum acquired from a 55 µm selected area from the opened seal of the chip packet. This was determined to be a qualitative match to the reference spectrum for high-density polyethylene (HDPE).
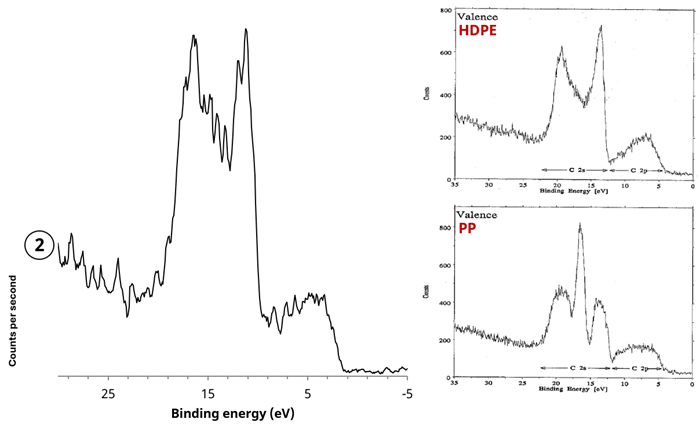
Figure 8. (left) X-ray excited valence band spectrum acquired from polymer region (2), (right) reference X-ray excited spectra from polyethylene (PE) and polypropylene (PP)2. Image Credit: Kratos Analytical, Ltd.
Conclusions
The experiment presented here highlighted the use of X-ray photoelectron spectroscopy and imaging in the characterization of a chip packet’s seal region after opening.
Results acquired show that forces involved in opening the packet trigger the delamination of multilayer materials under shear stress, with clear evidence that the barrier layer had failed as a result of brittleness during opening, with islands of passivated aluminum also observed.
References and Further Reading
- Sobhani, Z., et al. (2020). Microplastics generated when opening plastic packaging. Scientific Reports, (online) 10(1). https://doi.org/10.1038/s41598-020-61146-4.
- G. Beamson and D. Briggs. (1992) High resolution XPS of organic polymers: the Scienta ESCA300 database in SearchWorks catalog .New York: Wiley.(online) Available at: https://searchworks.stanford.edu/view/2407304 (Accessed 4 Sep. 2025).
- Strohmeier, B.R. (1990). An ESCA method for determining the oxide thickness on aluminum alloys. Surface and Interface Analysis, 15(1), pp.51–56. https://doi.org/10.1002/sia.740150109.
- Carlson, T.A. and McGuire, G.E. (1972). Study of the x-ray photoelectron spectrum of tungsten—tungsten oxide as a function of thickness of the surface oxide layer. Journal of Electron Spectroscopy and Related Phenomena, 1(2), pp.161–168. https://doi.org/10.1016/0368-2048(72)80029-x.
Acknowledgments
Produced from materials originally authored by Kratos Analytical Ltd.

This information has been sourced, reviewed and adapted from materials provided by Kratos Analytical, Ltd.
For more information on this source, please visit Kratos Analytical, Ltd.